Oral Session
Liver Imaging: Techniques
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| 09:15 | 0026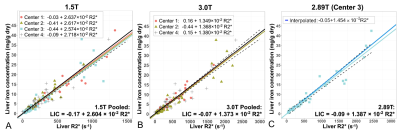 |
Multi-Center, Multi-Vendor Calibration and Validation of Liver Iron Quantification using R2*-MRI at 1.5T and 3T
Diego Hernando1, Ruiyang Zhao1, Qing Yuan2, Mounes Aliyari Ghasabeh3, Stefan Ruschke4, Xinran Miao1, Dimitrios C. Karampinos4, Lu Mao1, David T. Harris1, Ryan J. Mattison1, Michael R. Jeng5, Ivan Pedrosa2, Ihab R. Kamel3, Shreyas Vasanawala5, Takeshi Yokoo2, and Scott B. Reeder1
1University of Wisconsin-Madison, Madison, WI, United States, 2University of Texas Southwestern Medical Center, Dallas, TX, United States, 3The Johns Hopkins University, Baltimore, MD, United States, 4Technical University of Munich, Munich, Germany, 5Stanford University, Palo Alto, CA, United States We report the final results of an NIH funded study of MRI-based quantification of liver iron. R2* MRI enables quantification of liver iron concentration (LIC), but the cross-vendor differences of R2*-based LIC estimation remain unknown. Therefore, we evaluated the performance of R2*-based LIC via a single-breath-hold, confounder-corrected R2*-MRI at both 1.5T and 3T, through a multi-center, multi-vendor study. We confirmed a linear relationship between R2* and LIC, with highly similar calibrations across centers and vendors. Calibrations for 1.5T and 3T were generated. The data generated in this study provide the necessary multi-center calibrations for broad dissemination of R2*-based LIC quantification. |
|
09:27 |
0027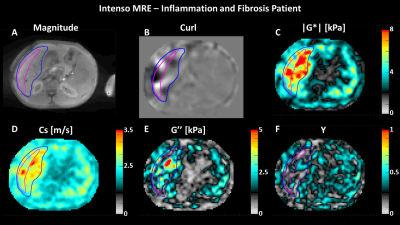 |
Simultaneous gauging of liver fibrosis and inflammation using 3D single breath-hold Intenso MRE with the gravitational transducer.
Omar Isam Darwish1,2,3, Radhouene Neji1,3, Sami Jeljeli1, Nader S Metwalli4, Jenna Feeley4, Yaron Rotman4, Rebecca J Brown4, Marc Ghany4, David E Kleiner5, Daniel Staeb6, Peter Speier7, Ralph Sinkus1,2, and Ahmed M Gharib4
1King's College London, London, United Kingdom, 2INSERM U1148, LVTS, University Paris Diderot, Paris, France, 3MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom, 4National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, United States, 5National Cancer Institute, Bethesda, MD, United States, 6MR Research Collaborations, Siemens Healthcare Limited, Melbourne, Australia, 7MR Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany Successful deployment of 3D liver MRE in the clinical assessment of fibrosis and inflammation requires a single breath-hold sequence to avoid geometric misalignment between different breath-holds and a phase-stable and efficient vibration for adequate wave penetration. We show initial data of Intenso MRE estimating the shear wave speed (Cs [m/s]) and the loss modulus (G’’ [kPa]). Both Cs and G’’ reflect a dependency on Ishak fibrosis and inflammation scores, respectively, in patients with chronic liver disease. In addition, we compare the magnitude of the shear modulus (|G*|) measured with Intenso and to the commonly utilized SE-EPI in such patients. |
|
09:39 |
0028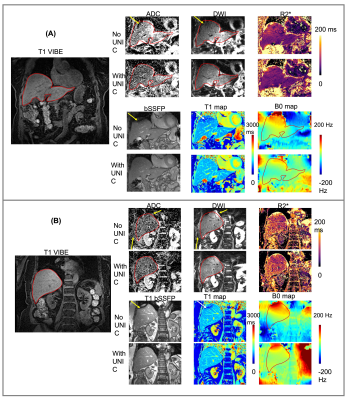 |
Integrated high-order B0 shimming for multiparametric quantitative liver imagingat 3T using a UNIfied Coil (UNIC)
Nan Wang1, Fardad M. Serry2, Michael Ocasio2, Yibin Xie2, Yuheng Huang2, Xinqi Li2, Pei Han2, Tianle Cao2, Sen Ma2, Fei Han3, Matthew Minton2, Yubin Cai2, Yujie Shan2, Xiaoming Bi3, Anthony G. Christodoulou2, Hsin-Jung Yang2, Debiao Li2, and Hui Han2
1Stanford University, Stanford, CA, United States, 2Cedars-Sinai Medical Center, Los Angeles, CA, United States, 3Siemens Medical Solutions, Los Angeles, CA, United States
Liver MRI shows promise for rich morphologic and physiologic information. B0 inhomogeneity causes off-resonance spread at the liver-lung interfaces, degrading image quality and quantification accuracy for DWI, T1, and T2*/R2*. A novel high-order shim coil (UNIC) was built, minimizing the decoupling between the two overlapped shim and RF arrays, allowing the free design of shim loop topology in proximity of the target organ. Improved shimming from UNIC is demonstrated with increased FOV in liver imaging, showing significantly reduced distortion in liver DWI, increased image quality score of MOLLI T1 map, and reduced B0-offset-induced ADC, R2*, and B0 standard deviations.
|
|
| 09:51 | 0029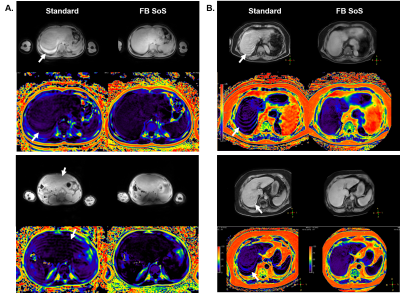 |
Free-breathing liver fat quantification with combined multi-echo Dixon and 3D radial stack-of-stars in children and adults at 3T and 1.5T
Shuo ZHANG1,2, Teresa Nolte2, Masami Yoneyama3, Sven Nebelung2, Christiane Kuhl2, Alexandra Barabasch2, and Jochen Herrmann4
1Philips GmbH Market DACH, Hamburg, Germany, 2Diagnostic and Interventional Radiology, University Hospital RWTH Aachen, Aachen, Germany, 3Philips Japan, Tokyo, Japan, 4Department of Diagnostic and Interventional Radiology and Nuclear Medicine, Section of Pediatric Radiology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Multi-echo gradient-echo Dixon based on the chemical shift method is currently the standard for non-invasive in vivo quantification of the liver fat but requires breath-holding. In young children or sick patients this is often challenging or not feasible. Despite recent attempts using different free-breathing approaches to mitigate patient motions, these are not widely available or routinely used. In this work we employ the multi-echo Dixon sequence with 3D pseudo-golden angle radial stack-of-stars readout for free-breathing liver fat assessment and report our initial results in children and adults at 3T and 1.5T with phantom validation and comparison to the standard method.
|
|
10:03 |
0030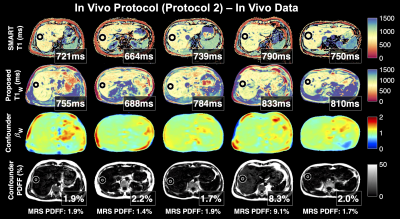 |
Confounder-Corrected T1 mapping in the Liver through Simultaneous Estimation of T1, PDFF, R2* and B1
Nathan Tibbitts Roberts1,2, Daiki Tamada, PhD1, Yavuz Muslu1,3, Diego Hernando, PhD1,3,4,5, and Scott B Reeder, MD, PhD1,3,4,6,7
1Radiology, University of Wisconsin - Madison, Madison, WI, United States, 2Electrical Engineering, University of Wisconsin - Madison, Madison, WI, United States, 3Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 4Biomedical Engineering, University of Wisconsin - Madison, Madison, WI, United States, 5Electrical and Computer Engineering, University of Wisconsin - Madison, Madison, WI, United States, 6Medicine, University of Wisconsin - Madison, Madison, WI, United States, 7Emergency Medicine, University of Wisconsin - Madison, Madison, WI, United States
T1 is emerging as a quantitative MR biomarker of liver fibrosis, the most important histological change predictive of progressing chronic liver disease. 3D spoiled gradient echo (SGRE)-based T1 mapping is preferable in the abdomen for its short acquisition time, volumetric coverage, and robustness to motion. However, SGRE-based T1 mapping is confounded by tissue fat and B1 inhomogeneity. In this work we present a novel SGRE-based T1 mapping method that corrects for both fat and B1 inhomogeneity. Phantom experiments show excellent agreement with reference T1 values (slope=1.01, R2=1.00) and minimal bias (~5±23ms). Early results from 5 healthy volunteers are promising.
|
|
| 10:15 | 0031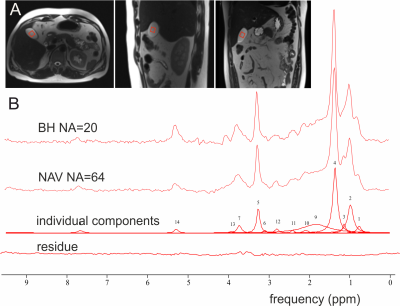 |
In vivo measurement of bile metabolite concentrations at 3T by 1H STEAM MRS – breathing triggered navigation vs breath-hold acquisition.
Ivica Just1,2, Michael Leutner2, Stefan Wampl3, Radka Klepochová2, Siegfried Trattnig1,4,5, Alexandra Kautzky-Willer2, and Martin Krššák2
1High-Field MR Centre, Department of Biomedical Imaging and Image-Guided Therapy, Medical University of Vienna, Vienna, Austria, 2Department of Internal Medicine III, Medical University of Vienna, Vienna, Austria, 3High-Field MR Center, Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Vienna, Austria, 4CD laboratory for Clinical Molecular MR imaging (MOLIMA), Vienna, Austria, 5Institute for Clinical Molecular MRI in the Musculoskeletal System, Karl Landsteiner Society, St. Pölten, Austria
Detection of metabolites from human gallbladder poses some challenges due to its size and position. We compared breath-hold and respiratory triggered acquisition by 1H SVS STEAM sequence on patients in supine position at 3T. 14 peaks of bile components were detected using both acquisition setups. Quantification of metabolite concentrations, corrected with measured T2app relaxation times, showed results within the range of previously published data. STEAM sequence proved to be feasible for measurement of bile components at 3T in vivo, providing sufficient spectral quality for metabolite quantification in supine position with comparable results in both breath-hold and PACE navigated acquisition.
|
|
| 10:27 | 0032 |
Spatial Scaling of Respiratory-Triggered Liver Diffusion Weighted Imaging
Johannes K. J. Raspe1,2, Anh T. Van1, Felix Harder1, Sean McTavish1, Johannes Peeters3, Kilian Weiss4, Marcus R. Makowski1, Rickmer F. Braren1, and Dimitrios C. Karampinos1
1Department of Diagnostic and Interventional Radiology, School of Medicine, Technical University of Munich, Munich, Germany, 2Department of Physics, Technical University of Munich, Garching, Germany, 3Philips Healthcare, Eindhoven, Netherlands, 4Philips GmbH Market DACH, Hamburg, Germany
Physiological motion induces signal loss in diffusion-weighted imaging of the liver even when respiratory triggering is employed. Cardiac motion mostly affects the left liver lobe resulting in an inhomogeneous signal appearance and overestimation of the apparent diffusion coefficient (ADC). Spatial scaling is an algorithm that handles the intrinsic weighting of signal by rejecting bad acquisitions and voxels with subsequent calculation of a spatially dependent scaling factor for the final averaging. The present work shows that by adding such a spatial scaling postprocessing step, liver signal homogeneity can be increased, and overestimation of the ADC can be reduced.
|
|
10:39 |
0033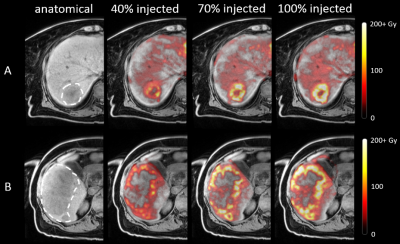 |
Intraprocedural MRI-based dosimetry during transarterial radioembolization with holmium-166 microspheres: a pilot study
Joey Roosen1, Lovisa E. L. Westlund Gotby1, Mark J. Arntz1, Jurgen J. Fütterer1, Marcel J. R. Janssen1, Meike W. M. van Wijk1, Christiaan G. Overduin1, and J. Frank W. Nijsen1
1Department of Medical Imaging, Radboud University Medical Center, Nijmegen, Netherlands
Transarterial radioembolization (TARE) is a treatment for liver tumors based on injection of radioactive microspheres in the hepatic arteries under angiography guidance. Conventionally, there is no feedback on the dose distribution during treatment and dosimetry can only be evaluated after treatment. As holmium-166 microspheres used for TARE can be quantified with MRI, we investigated the feasibility and safety of performing TARE in a 3-T MRI in six patients. Multi-echo gradient echo imaging was performed at set time points during administration and provided intraprocedural insight into the microsphere distribution. Our findings may prove useful for providing a personalized approach to TARE.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.