Oral Session
Hearty Talks: Cardiac Tissue Characterization
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| 14:30 | 0270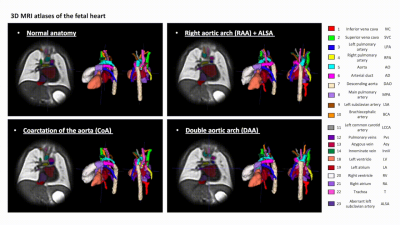 |
3D MRI atlases of congenital aortic arch anomalies and normal fetal heart: application to automated multi-label segmentation
Alena Uus1, Milou P.M. van Poppel1,2, Johannes K. Steinweg1, Irina Grigorescu1, Alexia Egloff Collado3, Paula Ramirez Gilliland1, Thomas A. Roberts1, Joseph V. Hajnal1,3, Mary Rutherford3, David F.A. Lloyd1,2, Kuberan Pushparajah1,2, and Maria Deprez1
1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Department of Congenital Heart Disease, Evelina London Children’s Hospital, London, United Kingdom, 3Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
This work introduces the first black blood 3D T2w MRI atlases of the normal fetal heart and congenital aortic arch anomalies. The atlases were generated from 87 subjects from normal, CoA, RAA and DAA cohorts and also include multi-label segmentations of the major cardiovascular structures. We further evaluated the feasibility of using deep learning for automated multi-label vessel segmentation in 3D T2w motion-corrected MRI images of the fetal heart.
|
|
| 14:42 | 0271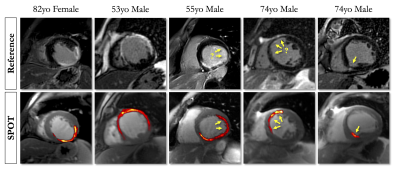 |
Improved Myocardial Scar Visualization with Two-minute Free-breathing Joint Bright- and Black-blood Late Gadolinium Enhancement Imaging
Aurelien Bustin1,2,3, Soumaya Sridi2, Aurelien Maillot1, Xavier Pineau2, Marta Nuñez-Garcia1, Maxime Sermesant1,4, Dounia El Hamrani1, Julie Magat1, Jérôme Naulin1, Stéphanie Clément-Guinaudeau2, Claire Bazin2, Gäel Dournes2, Michel Montaudon2, François Laurent2, Pierre Jaïs1,5, Matthias Stuber1,3,6, and Hubert Cochet1,2
1IHU LIRYC, Electrophysiology and Heart Modeling Institute, Université de Bordeaux – INSERM U1045, Bordeaux, France, 2Department of Cardiovascular Imaging, Hôpital Cardiologique du Haut-Lévêque, CHU de Bordeaux, Bordeaux, France, 3Department of Diagnostic and Interventional Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 4INRIA, Université Côte d’Azur, Sophia Antipolis, France, 5Department of Cardiac Electrophysiology, Hôpital Cardiologique du Haut-Lévêque, CHU de Bordeaux, Bordeaux, France, 6CIBM Center for Biomedical Imaging, Lausanne, Switzerland
Black-blood late gadolinium enhancement (LGE) techniques are increasingly being used to uncover myocardial scar patterns that may be otherwise confused with blood signal on conventional bright-blood LGE, especially when involving the subendocardium. With signal-attenuated blood and dark muscle representation, these techniques may however be hampered by a lack of anatomical information, making spatial localization of myocardial injuries a challenging task. Here we aim to assess the clinical performance of a novel LGE technique that combines both bright- and black-blood imaging in a single time-efficient free-breathing exam to obtain LGE images with unprecedented scar contrast and localization.
|
|
| 14:54 | 0272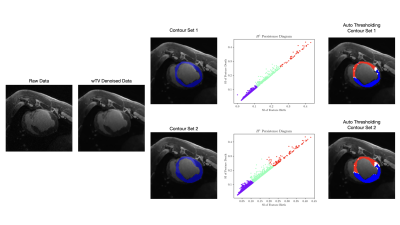 |
Validation of Automated Topological LGE Thresholding for Peri-Infarct Substrate Characterization
Calder David Sheagren1,2, Terenz Escartin1,2, Philippa Krahn1,2, Jaykumar Patel1,2, Fumin Guo2, and Graham Wright1,2
1Medical Biophysics, University of Toronto, Toronto, ON, Canada, 2Schulich Heart Centre, Sunnybrook Research Institute, Toronto, ON, Canada
High-resolution late gadolinium enhancement imaging is a powerful tool for arrhythmia risk assessment post-myocardial infarction, but requires substantial operator time and expertise to analyze. To address this challenge, automated analysis is introduced to isolate and depict relevant image features corresponding to healthy myocardium, peri-infarct gray zone, and dense scar. Using two sets of manual epicardial and endocardial contours, weighted total variation denoising is used to correct for statistical noise, and persistent homology is used to stratify topological features of the image. K-means clustering was used to generate remote myocardium and dense scar signal intensities for automated FWHM thresholding.
|
|
15:06 |
0273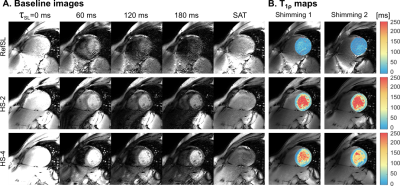 |
Adiabatic T1ρ mapping of the human myocardium at 3T.
Chiara Coletti1, Joao Tourais1, Telly Ploem1, Mehmet Akçakaya2, Christal van de Steeg-Henzen3, and Sebastian Weingärtner 1
1Department of Imaging Physics, TU Delft, Delft, Netherlands, 2Department of Electrical and Computer Engineering, University of Minnesota, Minneapolis, MN, United States, 3Medical Imaging and Radiation, Holland Proton Therapy Center, Delft, Netherlands
T1ρ-mapping constitutes a promising contrast-free alternative to LGE MRI for assessment of myocardial viability. However its applicability is hindered by susceptibility to B0 and B1+ field imperfections. In this work we propose a single breath-hold ECG-triggered adiabatically-prepared single-shot bSSFP T1ρ-mapping sequence. Bloch simulations are employed to identify optimal parameters for adiabatic pulses. The use of adiabatic preparations yields better resilience to system inhomogeneities and improved myocardium/blood contrast compared with conventional spin-lock modules, both in phantom and in-vivo. Thus, adiabatically-prepared sequences at 3T form a promising candidate for non-contrast evaluation of ischemic and non-ischemic cardiomyopathies.
|
|
15:18 |
0274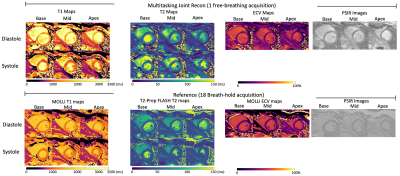 |
3D Joint Reconstruction of Non-Contrast and Contrast-Enhanced CMR Multitasking
Xianglun Mao1, Hsu-Lei Lee1, Alan C Kwan1,2, Tianle Cao1,3, Fei Han4, Yibin Xie1, Debiao Li1,3, and Anthony Christodoulou1,3
1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 3Department of Bioengineering, University of California in Los Angeles, Los Angeles, CA, United States, 4Siemens Medical Solutions USA, Los Angeles, CA, United States
Cardiovascular MR (CMR) is used to acquire function, morphology, and composition of the heart which aids in clinical diagnosis and management. While measures such as ejection fraction, ventricular dimensions, and presence/pattern of late gadolinium enhancement (LGE) have stood the test of time as diagnostic pillars, newer quantitative tissue property measurements such as T1, T2, and extracellular volume (ECV) can characterize diffuse fibrosis, inflammation, or infiltration. The purpose of this study was to integrate multiple quantitative measurements into a single 20-min free-breathing, non-ECG, motion-resolved whole ventricular 3D acquisition using CMR Multitasking, including cardiac function, pre-contrast T1/T2, ECV, and LGE images.
|
|
| 15:30 | 0275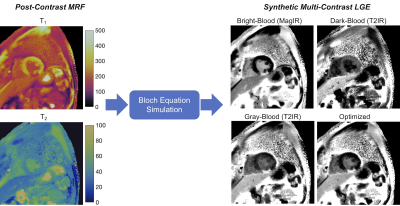 |
Synthetic Multi-Contrast Late Gadolinium Enhancement Using Post-Contrast Cardiac MR Fingerprinting
Imran Rashid1,2, Varun Rajagopalan1,2, Sadeer Al-Kindi1,2, Sanjay Rajagopalan1,2, Nicole Seiberlich3,4, and Jesse Ian Hamilton3,4
1Cardiology, University Hospitals Cleveland Medical Center, Cleveland, OH, United States, 2Medicine, Case Western Reserve University, Cleveland, OH, United States, 3Radiology, University of Michigan, Ann Arbor, MI, United States, 4Biomedical Engineering, University of Michigan, Ann Arbor, MI, United States
A technique is introduced using post-contrast cardiac Magnetic Resonance Fingerprinting (MRF) T1 and T2 maps to calculate synthetic late gadolinium enhancement (LGE) images with multiple contrast weightings, including bright-blood magnitude inversion recovery images, dark-blood T2-prepared inversion recovery images, and a novel contrast weighting (optimized for each patient) designed to improve differentiation among viable myocardium, blood, and scar based on measured T1 and T2 values. Initial results are presented in fifteen patients with ischemic cardiomyopathy scanned at 1.5T using conventional LGE and MRF-derived multi-contrast LGE images.
|
|
15:42 |
0276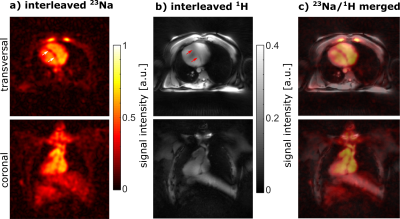 |
Interleaved 23Na/1H MRI of the human heart at 7 Tesla
Laurent Ruck1, Tobias Wilferth1, Lena V. Gast1, Tanja Platt2, Denise Lichthardt1, Christian R. Meixner1, Andreas K. Bitz3, Titus Lanz4, Simon Konstandin5, Christoph Kopp6, Michael Uder1, and Armin M. Nagel1,2
1Institute of Radiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 2Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 3Faculty of Electrical Engineering and Information Technology, University of Applied Sciences, Aachen, Germany, 4Rapid Biomedical GmbH, Rimpar, Germany, 5Fraunhofer MEVIS, Bremen, Germany, 6Department of Nephrology and Hypertension, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany A dual-nuclear interleaved 23Na/1H MRI sequence for cardiac MRI was implemented and evaluated in phantom and in vivo measurements using a 23Na body coil in combination with two 4 Tx/8 Rx 1H arrays. The 1H arrays were operated in 1Tx mode with fixed transmit magnitude/phase setting. Compared to single-nuclear sequences, the interleaved sequence led to almost identical SNR und image intensities in phantom measurements. Furthermore, the feasibility of interleaved 23Na/1H in vivo MRI measurements at 7 T was demonstrated. The interleaved approach enables reduced acquisition times and further eliminates the need for image co-registration. |
|
15:54 |
0277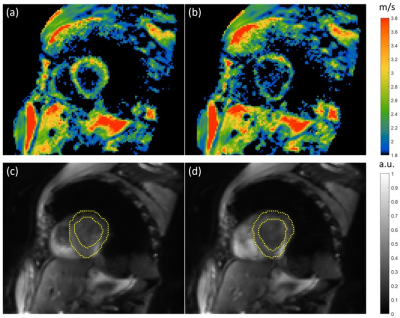 |
Multifrequency MR elastography for time-resolved shear wave speed quantification of the in-vivo human heart.
Matthias Anders1, Carsten Warmuth1, Heiko Tzschätzsch1, Helge Herthum1, Katja Degenhardt2, Sebastian Schmitter2, Jeanette Schulz-Menger1,3,4, Jürgen Braun1, and Ingolf Sack1
1Charité Universitätsmedizin Berlin, Berlin, Germany, 2Physikalisch-Technische Bundesanstalt(PTB), Braunschweig and Berlin, Berlin, Germany, 3Experimentaland Clinical Research Center (ECRC), DZHK partner site Berlin, Berlin, Germany, 4HELIOS Klinikum Berlin Buch, Berlin, Germany
Cardiac MR elastography (MRE) has the potential to noninvasively characterize the underlying pathophysiology in heart failure with preserved ejection fraction based on abnormal stiffness values. However, the need for synchronizing MRE with cardiac motion, breathing and harmonic vibrations continues to be a challenge. We have developed a single-shot, spin-echo cardiac MRE sequence, which continuously acquires wave images at multiple frequencies with retrospective gating of wave phases relative to the cardiac phase to cover the propagation of shear waves over the full cardiac cycle. Our preliminary data show the dispersion of myocardial stiffness over frequency in systole and diastole.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.