Oral Session
Prediction Tools for Dementia
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| 17:00 | 0717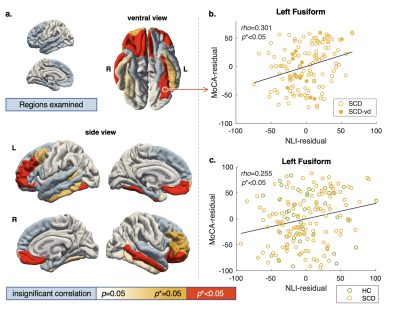 |
Frontal and Temporal Cortical Myelination Associations with Cognition Revealed by a Novel T1w/T2w-based Marker in Subjective Cognitive Decline
Yu Veronica Sui1, Arjun V Masurkar2,3,4, Karyn Marsh2, Barry Reisberg5, Thomas Wisniewski2,3,5,6, Henry Rusinek1,5, and Mariana Lazar1
1Department of Radiology, New York University Grossman School of Medicine, New York, NY, United States, 2Center for Cognitive Neurology, Department of Neurology, New York University Grossman School of Medicine, New York, NY, United States, 3Neuroscience Institute, New York University Grossman School of Medicine, New York, NY, United States, 4Department of Neuroscience and Physiology, New York University Grossman School of Medicine, New York, NY, United States, 5Department of Psychiatry, New York University Grossman School of Medicine, New York, NY, United States, 6Department of Pathology, New York University Grossman School of Medicine, New York, NY, United States
Identifying early changes associated with Alzheimer’s Disease (AD) and separating pathological from normal brain aging is a key step in AD treatment development. It has been proposed that a neuropathological retrogenesis heralded by cortical demyelination underlies the behavioral retrogenesis characterizing AD. As one of the earliest behavioral manifestations of AD, subjective cognitive decline (SCD) is a major research target, however, few studies have discovered reliable neural correlates at this stage. Here, using a novel cortical profile approach, we examined the cortical gradient of T1w/T2w, a putative myelin marker, in SCD and healthy control participants and its association with cognition.
|
|
| 17:12 | 0718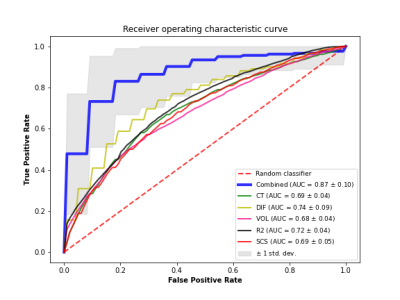 |
Towards an MRI-based Prediction of Neurofibrillary Tangles
Khalid Saifullah1, Mahir Tazwar1, Arnold M. Evia2, Ashish A. Tamhane2, David A. Bennett2, Julie A Schneider2, and Konstantinos Arfanakis1,2
1Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, United States, 2Rush Alzheimer's Disease Center, Rush University Medical Center, Chicago, IL, United States The purpose of this work was to develop an MRI-based classifier of neurofibrillary tangles by combining ex-vivo MRI and detailed neuropathology in brain autopsies from a large number (N=878) of community-based older adults. The ex-vivo classifier was trained on volumetric, cortical thickness, subcortical shape, diffusion, and R2 measurements, as well as age and sex. The average AUC of the final classifier was 0.87. Future work will convert the ex-vivo classifier to in-vivo and test it in-vivo in independent cohorts of older adults. |
|
17:24 |
0719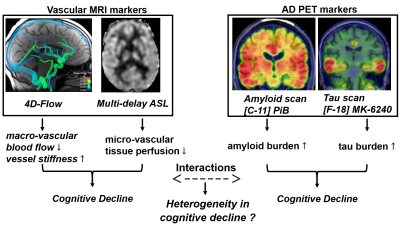 |
Examining cerebrovascular burden across the cognitive continuum in older adults with and without evidence of amyloidosis
Leonardo A Rivera-Rivera1, Karly A Cody1, Tobey A Betthauser1, Rebecca Koscik1, Erin Jonaitis 1, Robert Cadman1, Bruce Hermann1, Howard A Rowley1, Cynthia M Carlsson1, Nathaniel Chin1, Laura Eisenmenger1, Sterling C Johnson1, and Kevin M Johnson1
1University of Wisconsin, Madison, Madison, WI, United States In this study we investigated cerebrovascular health and vascular contributions to cognitive impairment in the presence and absence of amyloidosis. We employed various neuroimaging techniques including 4D-Flow, multi-delay ASL, T2-FLAIR, and structural T1 for vascular and structural biomarkers. β-amyloid (Aβ) burden was determined from PET imaging data. Data supports the notion that vascular dysfunction occurs in the presence and absence of Aβ, albeit with differing manifestations leading to cognitive decline. |
|
| 17:36 | 0720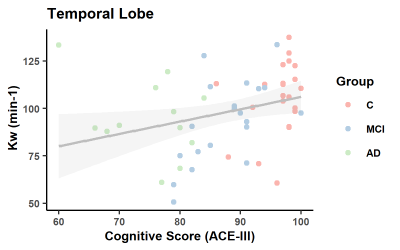 |
Blood-brain barrier water exchange rate is associated with cognitive performance in mild cognitive impairment and early Alzheimer’s disease
Catherine A Morgan1,2,3, Xingfeng Shao4, Jane Govender2, Tabitha Manson5, Vinod Suresh5,6, Deidre Jansson7, Danny JJ Wang4, David L Thomas8,9, Lynette Tippett1,2, and Michael Dragunow7
1School of Psychology and Centre for Brain Research, University of Auckland, Auckland, New Zealand, 2Brain Research New Zealand - Rangahau Roro Aotearoa, Centre of Research Excellence, New Zealand, Auckland, New Zealand, 3Centre for Advanced MRI, University of Auckland, Auckland, New Zealand, 4Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 5Auckland Bioengineering Institute, University of Auckland, Auckland, New Zealand, 6Department of Engineering Science, University of Auckland, Auckland, New Zealand, 7Department of Pharmacology and Centre for Brain Research, University of Auckland, Auckland, New Zealand, 8Department of Brain Repair and Rehabilitation, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom, 9Dementia Research Centre, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom
Blood-brain barrier (BBB) dysfunction has been observed in multiple neurodegenerative conditions, including mild cognitive impairment (MCI) and Alzheimer’s disease (AD). However recent concerns on the repeated use of Gadolinium based contrast agents (GBCAs), prompted us to investigate alternative, non-invasive methods for measuring BBB health. Diffusion-prepared arterial spin labelling (DP-ASL) imaging was implemented at 3T to determine water exchange rates (Kw) in 55 participants, comprising MCI, early AD and control participants. We found Kw to be associated with cognitive performance, suggesting it may be a useful imaging biomarker of early AD pathology.
|
|
| 17:48 | 0721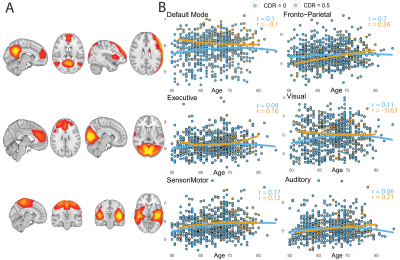 |
Multimodal MRI-derived phenotypes in preclinical Alzheimer’s Disease: results from the EPAD cohort
Luigi Lorenzini1, Silvia Ingala1, Alle Meije Wink1, Joost PA Kuijer 1, Viktor Wottschel 1, Carole H Sudre2,3,4,5, Sven Haller6,7, José Luis Molinuevo 8,9,10,11, Juan Domingo Gispert 8,10,11,12, David M Cash3, David L Thomas 13, Sjoerd B Vos 4,13, Prados Ferran14,15,16, Jan Petr17, Robin Wolz18, Alessandro Palombit18, Adam J Schwarz 19, Gael Chételat 20, Pierre Payoux 21,22, Carol Di Perri23,
Cyril Pernet 23, Giovanni Frisoni24,25, Nick C Fox 3, Craig Ritchie26, Joanna Wardlaw 23,27, Adam Waldman23,28, Frederik Barkhof1,29, and Henk JMM Mutsaerts 1,30
1Dept. of Radiology and Nuclear Medicine, Amsterdam University Medical Centre, Vrije Universiteit, Amsterdam Neuroscience, Amsterdam, The Netherlands, Amsterdam, Netherlands, 2MRC unit for Lifelong Health and Ageing at UCL, London, UK, London, United Kingdom, 3Dementia Research Centre, Department of Neurodegenerative Disease, UCL Queen Square Institute of Neurology, London, UK, London, United Kingdom, 4Centre for Medical Image Computing, University College London, London, UK, London, United Kingdom, 5School of Biomedical Engineering & Imaging Sciences, King’s College London, UK, London, United Kingdom, 6CIRD Centre d’Imagerie Rive Droite, Geneva, Switzerland, Geneva, Switzerland, 7Department of Surgical Sciences, Radiology, Uppsala University, Uppsala, Sweden, Uppsala, Sweden, 8Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain, Barcelona, Spain, 9CIBER Fragilidad y Envejecimiento Saludable (CIBERFES), Madrid, Spain, Madrid, Spain, 10IMIM (Hospital del Mar Medical Research Institute), Barcelona Spain, Barcelona, Spain, 11Universitat Pompeu Fabra, Barcelona, Spain, Barcelona, Spain, 12CIBER Bioingeniería, Biomateriales y Nanomedicina (CIBER-BBN), Madrid, Spain, Madrid, Spain, 13Neuroradiological Academic Unit, UCL Queen Square Institute of Neurology London, UK, London, United Kingdom, 14Nuclear Magnetic Resonance Research Unit, Queen Square Multiple Sclerosis Centre, University College London Institute of Neurology, London, United Kingdom, London, United Kingdom, 15Department of Medical Physics and Biomedical Engineering, Centre for Medical Image Computing, University College London, London, United Kingdom, London, United Kingdom, 16e-Health Centre, Open University of Catalonia, Barcelona, Spain, Barcelona, United Kingdom, 17Helmholtz‐Zentrum Dresden‐Rossendorf, Institute of Radiopharmaceutical Cancer Research, Dresden, Germany, Dresden, Germany, 18IXICO, London, UK, London, United Kingdom, 19Takeda Pharmaceuticals Ltd., Cambridge, MA, USA, Cambridge, ME, United States, 20Université de Normandie, Unicaen, Inserm, U1237, PhIND "Physiopathology and Imaging of Neurological Disorders", institut Blood-and-Brain @ Caen-Normandie, Cyceron, 14000 Caen, France, Caen, France, 21Department of Nuclear Medicine, Toulouse CHU, Purpan University Hospital, Toulouse, France, Toulouse, France, 22Toulouse NeuroImaging Center, University of Toulouse, INSERM, UPS, Toulouse, France, Toulouse, France, 23Centre for Clinical Brain Sciences, The University of Edinburgh, Edinburgh, UK, Edinburgh, Scotland, 24Laboratory Alzheimer’s Neuroimaging & Epidemiology, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy, Brescia, Italy, 25University Hospitals and University of Geneva, Geneva, Switzerland, Geneva, Switzerland, 26Centre for Dementia Prevention, The University of Edinburgh, Scotland, UK, Edinburgh, Scotland, 27UK Dementia Research Institute at Edinburgh, University of Edinburgh, UK, Edinburgh, Scotland, 28Department of Medicine, Imperial College London, London, UK, London, United Kingdom, 29Institute of Neurology and Healthcare Engineering, University College London, London, UK, London, United Kingdom, 30Ghent Institute for Functional and Metabolic Imaging (GIfMI), Ghent University, Ghent, Belgium, Ghent, Belgium
Image-derived phenotypes (IDPs) from multimodal MRI sequences constitute an important resource that allows the characterization of brain alterations in the early stages of Alzheimer diseases and other neurodegenerative conditions. Here, we showed the computation of multimodal IDPs from the European Prevention of Alzheimer Dementia (EPAD) cohort and assessed their relationship with non-imaging markers of neurodegeneration. We demonstrated the clinical relevance of IDPs to uncover early brain alteration in AD by showing expected association with non-imaging data.
|
|
18:00 |
0722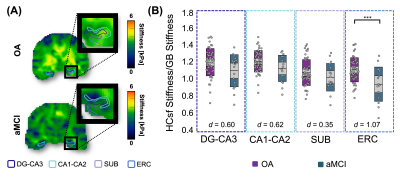 |
Hippocampal Subfield Property Differences in Amnestic Mild Cognitive Impairment Measured with MR Elastography
Peyton L Delgorio1, Lucy V Hiscox2, Grace McIlvain1, Alexis A Merritt1, Alexa M Diano1, Mary K Kramer1, Kyra E Twohy1, James M Ellison3, Alyssa Lanzi1, Matthew L Cohen1, Christopher R Martens1, and Curtis L Johnson1
1University of Delaware, Newark, DE, United States, 2University of Bath, Bath, United Kingdom, 3ChristianaCare Health System, Newark, DE, United States
The aim of this study was to determine whether magnetic resonance elastography (MRE) could sensitively detect mechanical property alterations in the hippocampal subfields due to amnestic mild cognitive impairment (aMCI) - a prodromal stage of Alzheimer’s disease (AD). Results show that entorhinal cortex viscoelasticity was significantly lower in aMCI participants. Further, the entorhinal cortex did not display significant volume differences due to aMCI, which demonstrates how MRE may yield more information about the health of a region known to harbor AD pathology. These results suggest that hippocampal subfield MRE measures show potential for use as an imaging biomarker of disease.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.