Oral Session
Preclinical & Task-Based fMRI
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| 16:45 | 0525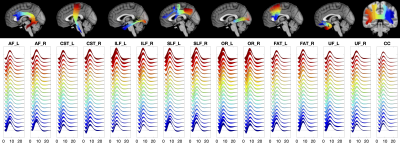 |
Along-tract quantification of the BOLD hemodynamic response function in white matter
Kurt G Schilling1, Muwei Li1, Francois Rheault2, Yurui Gao2, Zhaohua Ding2, Adam W Anderson2, Hakmook Kang2, Bennett A Landman2, and John C Gore1
1Vanderbilt University Medical Center, Nashville, TN, United States, 2Vanderbilt University, Nashville, TN, United States
We measured the variations of the BOLD hemodynamic response function (HRF) along white matter (WM) pathways. We find that the WM HRF is different to that of the gray matter (GM), and has a prominent negative dip and smaller peak signal. Further, we find that the HRF changes along WM pathways, and is different between pathways. Characterizing the variations of the WM HRF along and across pathways may provide insight into the biophysical basis of BOLD effects in WM and the relationships between WM structure and neurovascular coupling.
|
|
| 16:57 | 0526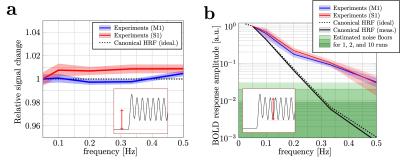 |
Tracking rapid stimulus-driven BOLD oscillations in the human primary motor cortex and somatosensory cortex
Shota Hodono1,2, Jonathan R Polimeni3,4, David Reutens1,2, and Martijn A Cloos1,2
1Centre for Advanced Imaging,The University of Queensland, Brisbane, Australia, 2ARC Training Centre for Innovation in Biomedical Imaging Technology, The University of Queensland, Brisbane, Australia, 3Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Harvard Medical School, Massachusetts General Hospital, Charlestown, MA, United States, 4Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States We investigated the observable frequency range of stimulus-driven BOLD oscillations in human M1 and S1. Experimental results showed that BOLD response oscillations up to 0.50 Hz can be measured in individual subjects. The responses were substantially higher than predicted using the canonical HRF model. Despite their |
|
17:09 |
0527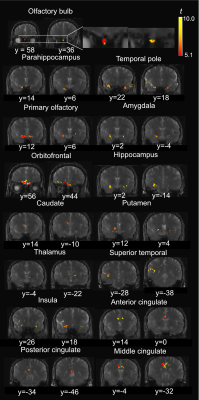 |
Functional activities detected in the olfactory bulb and associated olfactory regions in the human brain using T2-prepared BOLD fMRI at 7T Video Not Available
Xinyuan Miao1,2, Adrian G. Paez1,2, Suraj Rajan3, Di Cao1,2,4, Dapeng Liu1,2, Alex Y. Pantelyat3, Liana I. Rosenthal3, Peter C.M. van Zijl1,2, Susan S. Bassett5, David M. Yousem6, Vidyulata Kamath5, and Jun Hua1,2
1Neurosection, Division of MRI Research, Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4Department of Biomedical Engineering, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 5Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 6Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Conventional EPI-based BOLD fMRI can be challenging in olfaction related brain regions, such as the olfactory bulb (OB), mainly due to the large susceptibility artifacts. To date, few studies have demonstrated successful fMRI in the human OB. T2-prepared (T2prep) BOLD fMRI is an alternative approach developed especially for reducing such susceptibility artifacts. Here, olfactory fMRI was performed at 7T in 14 healthy participants. T2prep-BOLD showed greater functional sensitivity than GRE-EPI-BOLD in the OB and associated olfactory regions. Habituation effects and a bi-phasic pattern of fMRI signal changes were observed using T2prep-BOLD, which showed a good intra-subject reproducibility.
|
|
17:21 |
0528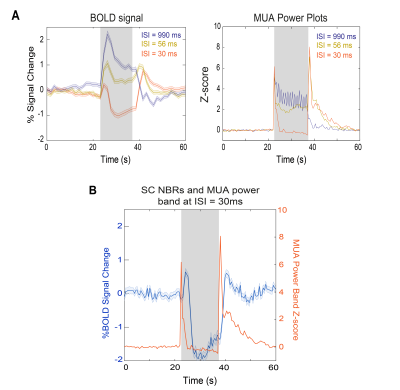 |
Negative BOLD closely follows neuronal suppression in superior colliculus
Rita Gil1, Mafalda Valente1, Alfonso Renart1, and Noam Shemesh1
1Champalimaud Research, Champalimaud Centre for the Unknown, Lisbon PT, Lisbon, Portugal
The underlying sources of negative BOLD responses (NBRs) are still debated. Here, we show rat superior colliculus (SC) NBRs associated with visual stimulation at short inter-stimulus intervals (ISIs) along with decreases in power of local field potentials and multi-unit activity signals measured in this region. This hints to neuronal suppression, possibly due to impossibility of complete excitability recovery upon short ISIs, associated with NBRs. Moreover, both NBRs and electrophysiological power time profiles reveal one peak after stimulus started and another when it ceased, highlighting the SC nature of detecting "brightness changes" when individual flashes are no longer perceivable.
|
|
| 17:33 | 0529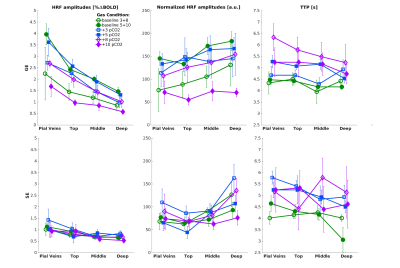 |
The Relative Contribution of the Vascular Architecture and Reactivity to the BOLD signal Formation
Emiel C.A. Roefs1, Wouter Schellekens1, Mario G. Báez-Yáñez1, Alex A. Bhogal1, Jeroen C.W. Siero1,2, and Natalia Petridou1
1Radiology department, Center for Image Sciences, UMC Utrecht, Utrecht, Netherlands, 2Spinoza Center for Neuroimaging, Amsterdam, Netherlands
In this study, we investigate the vascular contribution to the BOLD signal by comparing purely non-neuronal-related changes in the BOLD signal induced by gas manipulations with neuronal-related hemodynamic changes in the BOLD signal for different vascular compartments. Different vascular compartments were targeted by employing gradient-echo and spin-echo in combination with cortical depth estimations and pial vein segmentations. Our findings suggest that the increase in macro-vascular baseline venous blood volume (CBVv0) is the main contributor to the large GE-BOLD signal increase towards the pial surface and that normalization for this CBVv0-dependence is possible using a hyperoxia breathing task.
|
|
| 17:45 | 0530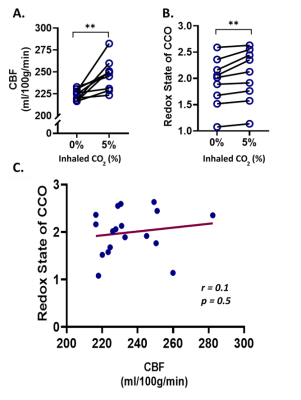 |
The relationship between cytochrome oxidase redox state and CBF is not constant: A multimodal 9.4T NIRS-MRI study on animal model
Mada Hashem1,2,3,4, Ying Wu2,3,4, and Jeff F. Dunn2,3,4
1Biomedical Engineering Graduate Program, University of Calgary, Calgary, AB, Canada, 2Department of Radiology, University of Calgary, Calgary, AB, Canada, 3Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada, 4Experimental Imaging Centre, University of Calgary, Calgary, AB, Canada Non-invasive imaging of cerebral oxygen delivery and consumption is crucial to understand neurovascular coupling and oxidative metabolism. We combine NIRS and MRI to determine the correlation between mitochondrial status and perfusion in the cortex of mice, when exposed to hypercapnia or varying oxygen levels. There was no correlation between perfusion and redox state under hypercapnia, while a strong correlation was observed under varying oxygen levels. This proves that the relationship between perfusion and mitochondrial redox can be quantified non-invasively in vivo. Such simultaneous measurements, in neurovascular diseases, will help determine whether there is abnormal neurovascular coupling or abnormal oxidative metabolism. |
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.