Oral Session
Antenatal, Neonatal & Pediatric MRI: Congenital & Acquired Insults in the Developing Brain
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| 09:27 | 0610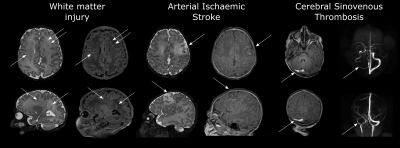 |
Risk Factors for Perioperative Brain Lesions on MRI in Infants with Severe Congenital Heart Disease: A European Collaboration Video Permission Withheld
Alexandra F Bonthrone1, Raymond Stegeman2,3,4,5,6, Maria Feldmann7, Nathalie HP Claessens2,3,4,6, Maaike Nijman2,3,4,6, Nicolaas JG Jansen3,8, Joppe Nijman3, Floris Groenendaal2,6, Linda S de Vries2, Manon JNL Benders2, Felix Haas5, Mireille N Bekker9, Thushiha Logeswaran10, Bettina Reich11, Raimund Kottke12, Cornelia Hagmann13, Bea Latal7, Hitendu Dave14, John Simpson15, Kuberan Pushparajah15,16,
Conal Austin15, Christopher J Kelly1, Sophie Arulkumaran1, Mary A Rutherford1, Serena J Counsell1, Walter Knirsch17, and Johannes MPJ Breur4
1Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Department of Neonatology, Wilhelmina Children’s Hospital, University Medical Center Utrecht and Utrecht University, Utrecht, Netherlands, 3Department of Pediatric Intensive Care, Wilhelmina Children’s Hospital, University Medical Center Utrecht and Utrecht University, Utrecht, Netherlands, 4Department of Pediatric Cardiology, Wilhelmina Children’s Hospital, University Medical Center Utrecht and Utrecht University, Utrecht, Netherlands, 5Congenital Cardiothoracic Surgery, Wilhelmina Children’s Hospital, University Medical Center Utrecht and Utrecht University, Utrecht, Netherlands, 6Utrecht Brain Center, University Medical Center Utrecht and Utrecht University, Utrecht, Netherlands, 7Child Development Center, University Children's Hospital Zurich, Zurich, Switzerland, 8Department of Pediatrics, Beatrix Children’s Hospital, University Medical Center Groningen, Groningen, Netherlands, 9Department of Obstetrics, Wilhelmina Children’s Hospital, University Medical Center Utrecht and Utrecht University, Utrecht, Netherlands, 10Pediatric Heart Center, University Hospital Giessen, Justus-Liebig-University Giessen, Giessen, Germany, 11Department of Congenital Heart Disease and Pediatric Cardiology, German Heart Center Munich, Technical University of Munich, Munich, Germany, 12Department of Diagnostic Imaging, University Children’s Hospital Zurich, Zurich, Switzerland, 13Department of Neonatology and Pediatric Intensive Care, University Children’s Hospital Zurich, Zurich, Switzerland, 14Division of Congenital Cardiovascular Surgery, Pediatric Heart Centre, University Children’s Hospital Zurich, Zurich, Switzerland, 15Paediatric Cardiology Department, Evelina London Children's Healthcare, London, United Kingdom, 16Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 17Pediatric Cardiology, Pediatric Heart Center, Department of Surgery, Children’s Research Center, University Children’s Hospital, and University of Zurich, Zurich, Switzerland
Infants with Congenital Heart Disease (CHD) are at risk of neurodevelopmental impairments. MRI studies have identified brain injury in infants with CHD both before and after cardiac surgery. We characterized risk factors for preoperative and new postoperative arterial ischaemic stroke (AIS), white matter injury (WMI) and cerebral sinovenous thrombosis (CSVT) in a cohort of infants with severe CHD. Balloon atrial septostomy was associated with increased risk of preoperative brain injury. Induced vaginal delivery was associated with preoperative WMI. Cardiac physiology and perioperative factors were associated with increased risk of new postoperative brain injury.
|
|
| 09:39 | 0611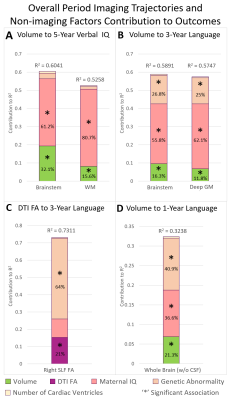 |
Early Postnatal Multi-modal MRI Structural Trajectory of Brain Dysmaturation Predicts Early Childhood Language Outcomes in Complex CHD
Vincent Kyu Lee1,2, Rafael Ceschin3,4, William Thomas Reynolds2,3, Benjamin Meyers4, Julia Wallace4, Douglas Landsittel5, Daryaneh Badaly6, J William Gaynor7, Daniel Licht8, Nathaniel H Greene9, Ken M Brady10, Jill V Hunter11, Zili D Chu12, Elisabeth A Wilde13, R Blaine Easley14, Dean Andropoulos14, and Ashok Panigrahy1,2,3,4
1Bioengineering, University of Pittsburgh, Pittsburgh, PA, United States, 2Radiology, UPMC Children's Hospital of Pittsburgh, Pittsburgh, PA, United States, 3Biomedical Informatics, University of Pittsburgh, Pittsburgh, PA, United States, 4Radiology, University of Pittsburgh, Pittsburgh, PA, United States, 5Epidemiology and Biostatistics, Indiana University School of Public Health-Bloomington, Bloomington, IN, United States, 6Learning and Development Center, Child Mind Institute, New York, NY, United States, 7Division of Cardiothoracic Surgery, Children’s Hospital of Philadelphia, Philadelphia, PA, United States, 8Division of Neurology, Children’s Hospital of Philadelphia, Philadelphia, PA, United States, 9Anesthesiology, Oregon Health and Sciences University, Portland, OR, United States, 10Anesthesiology, Lurie Children’s Hospital, Northwestern University, Chicago, IL, United States, 11Radiology, Texas Children's Hospital, Houston, TX, United States, 12Radiology, Texas Children's Hospital, Baylor College of Medicine, Houston, TX, United States, 13Neurology, University of Utah School of Medicine, Salt Lake City, UT, United States, 14Anesthesiology, Perioperative and Pain Medicine, Texas Children's Hospital, Baylor College of Medicine, Houston, TX, United States
This study examined trajectories of early postnatal brain structure (macrostructural brain volumes and microstructural white matter tractography) relationship with early childhood neurodevelopmental deficits (NDD) in complex congenital heart disease patients. This analysis included development of predictive multi-variable models incorporating other known risk factors of poor NDD in CHD. A multi-modal dysmaturation phenotype of reduced subcortical volume and cerebral white matter volume/connectivity predicted poor early childhood language outcomes, despite high relative contribution of genetic and socio-demographic factors (maternal IQ). Favorable socio-demographic factors, despite the high incidence of focal WMI and presence of genetic abnormalities, predicted better neurodevelopmental outcomes.
|
|
| 09:51 | 0612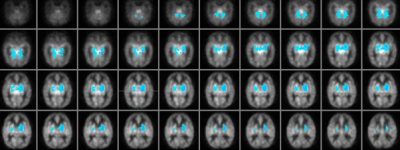 |
Hyperperfusion on arterial spin labelling is associated with cognitive impairment in infants with neonatal hypoxic ischemic encephalopathy
Ruth O'Gorman Tuura1, Raimund Kottke2, Barbara Brotschi3, Carola Sabandal4, Cornelia Hagmann3, and Beatrice Latal5
1Center for MR Research, University Children's Hospital, Zurich, Switzerland, 2Radiology, University Children's Hospital, Zurich, Switzerland, 3Neonatology and Pediatric Intensive Care, University Children's Hospital, Zurich, Switzerland, 4Anaesthesia, University Children's Hospital, Zurich, Switzerland, 5Child Development Center, University Children's Hospital, Zurich, Switzerland
Neonatal hypoxic ischemic encephalopathy (HIE) is a serious neurological condition, representing a primary cause of neonatal death and developmental impairment. In newborns with HIE, hyperperfusion is related to severe adverse outcomes, but less is known about the link between perfusion and mild to moderate developmental impairments. Using a voxelwise correlation analysis, we investigated the link between ASL perfusion in newborns with HIE and developmental outcome at 2 years. A more adverse outcome was associated with hyperperfusion across the whole brain. A better cognitive outcome was associated with lower perfusion in the bilateral basal ganglia, thalamus, hippocampus and cerebellum.
|
|
| 10:03 | 0613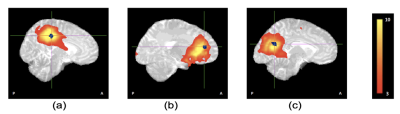 |
Resting-state functional connectivity reductions in the cingulate gyrus in HIV exposed uninfected neonates
Jia Fan1,2, Fleur Warton1,2, Samantha Fry3, Mark Cotton3, Sandra Jacobson1,4, Joseph Jacobson1,4, Christopher Molteno5, Francesca Little6, Andre van der Kouwe1,7,8, Barbara Laughton3, and Ernesta Meintjes1,2,9
1Biomedical Engineering Research Centre, Division of Biomedical Engineering, Department of Human Biology, University of Cape Town, Cape Town, South Africa, 2Neuroscience Institute, University of Cape Town, Cape Town, South Africa, 3Family Centre for Research with Ubuntu, Department of Paediatrics and Child Health and Tygerberg Children’s Hospital, Faculty of Medicine and Health Sciences, Stellenbosch University, Stellenbosch, South Africa, 4Department of Psychiatry and Behavioral Neurosciences, Wayne State University School of Medicine, Detroit, MI, United States, 5Department of Psychiatry and Mental Health, University of Cape Town, Cape Town, South Africa, 6Department of Statistical Sciences, University of Cape Town, Cape Town, South Africa, 7A.A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 8Department of Radiology, Harvard Medical School, Boston, MA, United States, 9Cape Universities Body Imaging Centre, University of Cape Town, Cape Town, South Africa
Aggressive combination antiretroviral treatment in pregnancy has significantly reduced new perinatal HIV infections giving rise to a growing population of HIV exposed uninfected (HEU) children. Children who are HEU demonstrate neurodevelopmental delay compared to their HIV-unexposed uninfected (HUU) peers. We examined resting-state functional connectivity (RSFC) in neonates exposed to HIV and ART in utero and perinatally using resting-state fMRI. Ten standard resting-state networks were identified from independent component analysis. Voxelwise group comparison between neonates who are HEU and HUU revealed localized RSFC reductions in the cingulate gyrus within 3 networks: medial somatosensory, and anterior and posterior default mode networks.
|
|
| 10:15 | 0614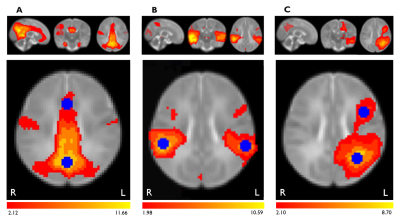 |
Impacts of prenatal COVID-19 pandemic-related distress on brain functional connectivity in 3-month infants
Aliza Jaffer1, Kathryn Y Manning2, Gerald F Giesbrecht 3, Lianne Tomfohr-Madsen3, and Catherine Lebel2
1University of Calgary, Calgary, AB, Canada, 2Department of Radiology, University of Calgary, Calgary, AB, Canada, 3Department of Pediatrics, University of Calgary, Calgary, AB, Canada Prenatal psychological distress has been substantially higher during the COVID-19 pandemic, but its impacts on infant brain development remain unclear. We investigated the relationship between prenatal pandemic-related distress and functional connectivity within the default mode (DMN), auditory, and left frontoparietal brain networks in 3-month old infants. Resting-state functional MRI scans were analysed using FSL. Higher prenatal anxiety was associated with increased infant functional connectivity within the DMN. These connectivity changes may predispose these infants to later mental health challenges, and highlight the need to screen for prenatal anxiety and provide support for pregnant individuals and infants born during the pandemic. |
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.