Oral Session
Young Investigator Awards
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| 09:15 | 0001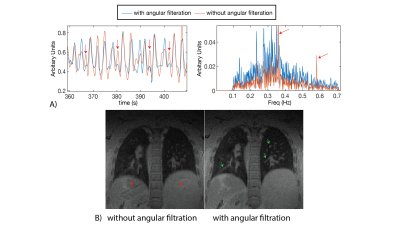 |
Self-gated 3D Stack-of-Spirals Ultra-Short Echo-Time Pulmonary imaging at 0.55T
Ahsan Javed1, Rajiv Ramasawmy1, Kendall O’Brien1, Christine Mancini1, Pan Su2, Waqas Majeed2, Thomas Benkert3, Himanshu Bhat2, Anthony F. Suffredini4, Ashkan Malayeri5, and Adrienne E Campbell-Washburn1
1Cardiovascular Branch, Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States, 2Siemens Medical Solutions USA Inc., Malvern, PA, United States, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Critical Care Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD, United States, 5Department of Radiology and Imaging Sciences, Clinical Center, Department of Health and Human Services, National Institutes of Health, Bethesda, MD, United States
High-performance 0.55T MRI is promising for lung imaging due to the reduced susceptibility artifacts. However, high-resolution lung imaging is still challenged by low proton density and SNR. 3D spiral acquisitions can be used to improve SNR-efficiency, but these readouts are susceptible to trajectory errors and blurring from concomitant-fields, which are amplified at lower field strengths. Here we present a self-gated, ultra-short echo-time, stack-of-spirals acquisition which leverages rapid inline corrections for trajectory imperfections, trajectory dependent navigator signal fluctuations, and concomitant-fields to enable robust 1.75mm isotropic lung imaging at 0.55T. We also demonstrate our technique in healthy-volunteers, patients with lung-nodules and COVID-19.
|
|
| 09:35 | 0002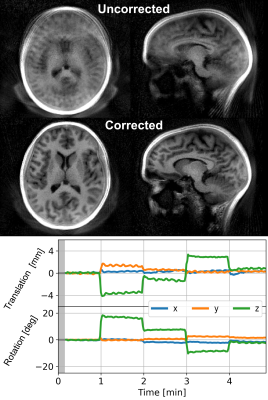 |
Motion Corrected Silent ZTE Neuroimaging
Emil Ljungberg1,2, Tobias C. Wood1, Ana Beatriz Solana3, Steven C.R. Williams1, Gareth J. Barker1, and Florian Wiesinger1,3
1Department of Neuroimaging, Institute of Psychiatry, Psychology, and Neuroscience, King’s College London, London, United Kingdom, 2Department of Medical Radiation Physics, Lund University, Lund, Sweden, 3GE Healthcare, Munich, Germany
We present a new method called MERLIN for motion corrected silent neuroimaging using zero echo time (ZTE) MRI. Self-navigation is achieved with an interleaved 3D spiral phyllotaxis trajectory to produce image navigators. Retrospective motion correction is then applied to the collected raw data. The acoustic noise of MELIN was less than 4 dBA above ambient levels. A range of different head motion paradigms were evaluated (rotation and nodding), showing greatly improved image quality after motion correction in all cases.
|
|
| 09:55 | 0003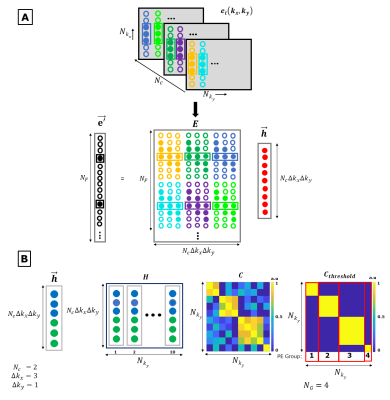 |
External Dynamic InTerference Estimation and Removal (EDITER) for low field MRI
Sai Abitha Srinivas1,2, Stephen F Cauley3,4, Jason P Stockmann3,4, Charlotte R Sappo1,2, Christopher E Vaughn1,2, Lawrence Wald3,4,5, William Grissom1,2,6,7, and Clarissa Cooley3,4
1Vanderbilt University Institute of Imaging Science, Nashville, TN, United States, 2Department of Biomedical Engineering, Vanderbilt University, Nashville, TN, United States, 3Harvard Medical School, Boston, MA, United States, 4Dept. of Radiology, Massachusetts General Hospital, Athinoula A Martinos Center for Biomedical Imaging, Boston, MA, United States, 5Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA, United States, 6Department of Electrical Engineering, Vanderbilt University, Nashville, TN, United States, 7Department of Radiology, Vanderbilt University, Nashville, TN, United States
Point of care MRI requires operation outside of an MR shielded room where electromagnetic interference can degrade image quality. To address this, we demonstrate a self-calibrated generalized dynamic model to retrospectively remove time-varying external interference. The method uses data acquired from multiple Electromagnetic Interference (EMI) detectors simultaneous with the primary MR coil. We test the approach both in a controlled EMI setting on an 80mT Halbach scanner and an uncontrolled real-world EMI setting on a 47.5mT permanent magnet open MRI system.
|
|
| 10:15 | 0004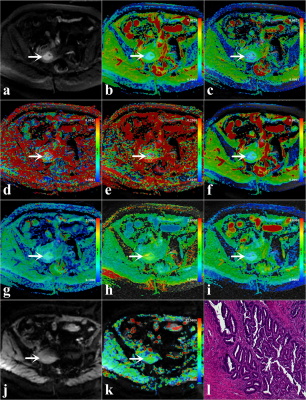 |
Amide proton transfer-weighted MRI with multiple models DWI facilitates preoperative risk stratification of early stage endometrial carcinoma
Nan Meng1,2, Ting Fang1,2, Pengyang Feng3, Zhun Huang3, Jing Sun4, Xuejia Wang5, Jie Shang6, Kaiyu Wang7, Dongming Han5, and Meiyun Wang1,2
1Department of Medical Imaging, Zhengzhou University People’s Hospital & Henan Provincial People’s Hospital, Zhengzhou, China, 2Academy of Medical Sciences, Zhengzhou University, Zhengzhou, China, 3Department of Medical Imaging, Henan University People’s Hospital & Henan Provincial People’s Hospital, Zhengzhou, China, 4Department of Pediatrics, Zhengzhou Central Hospital, Zhengzhou University, Zhengzhou, China, 5Department of MR, the First Affiliated Hospital, Xinxiang Medical University, Weihui, China, 6Department of Pathology, the First Affiliated Hospital, Xinxiang Medical University, Weihui, China, 7MR Research China, GE Healthcare, Beijing, China
Intravoxel incoherent motion (IVIM) and diffusion kurtosis imaging (DKI) can fully reflect the information of water molecular diffusion, microcirculation perfusion, and tissue heterogeneity. Amide proton transfer-weighted imaging (APTWI) has unique advantages in displaying mobile proteins and polypeptides. Our results showed that IVIM, DKI, and APTWI can estimate the risk stratification of early-stage (FIGO ≤ I) endometrial carcinoma (EC), and the combination of D, MK, and MTRasym (3.5 ppm) may be an effective imaging marker for identifying low-risk and non-low-risk early-stage EC.
|
|
| 10:35 | 0005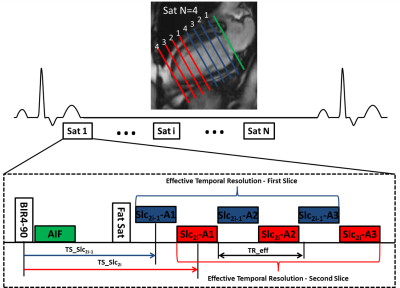 |
Diagnostic Accuracy of Spiral Whole-Heart Quantitative Adenosine Stress Cardiovascular Magnetic Resonance with Motion Compensated L1-SPIRIT
Jonathan A. Pan1, Austin A. Robinson2, Patricia Rodriguez Lozano1, Yang Yang3, Stephen McHugh4, Eric M. Holland5, Craig H. Meyer6,7, Angela M. Taylor1, Christopher M. Kramer1,7, and Michael Salerno8
1Cardiovascular Division, Department of Medicine, University of Virginia Health System, Charlottesville, VA, United States, 2Division of Cardiovascular Diseases, Division of Radiology, Scripps Clinic, La Jolla, CA, United States, 3Biomedical Engineering and Imaging Institute and Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 4Department of Internal Medicine, Lewis Katz School of Medicine at Temple University, Philadelphia, PA, United States, 5Division of Cardiology, Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 6Department of Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 7Department of Radiology and Medical Imaging, University of Virginia Health System, Charlottesville, VA, United States, 8Division of Cardiovascular Medicine, Stanford University, Palo Alto, CA, United States
Variable density spiral (VDS) pulse sequences with motion compensated compressed sensing reconstruction allow for whole-heart quantitative assessment of myocardial perfusion but have not yet been clinically validated. In this study, we showed that whole-heart VDS stress perfusion has good diagnostic accuracy and ischemic burden evaluation. No significant difference was seen between visual and quantitative diagnostic performance and ischemic burden measurements.
|
|
| 10:55 | 0006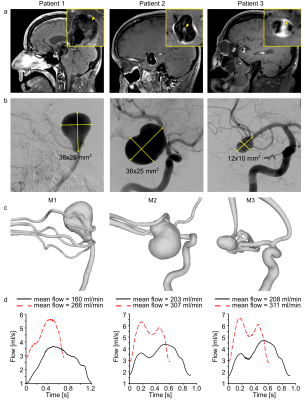 |
Pseudo-Enhancement in Intracranial Aneurysms on Black-Blood MRI: Effects of Flow Rate, Spatial Resolution, and Additional Flow Suppression.
Mariya S. Pravdivtseva1, Franziska Gaidzik2, Philipp Berg2, Carson Hoffman3, Leonardo A. Rivera-Rivera3, Rafael Medero4, Lindsay Bodart3, Alejandro Roldan-Alzate4, Michael A. Speidel3, Kevin M. Johnson3, Oliver Wieben3, Olav Jansen5, Jan-Bernd Hövener1,
and Naomi Larsen5
1Section Biomedical Imaging, Molecular Imaging North Competence Center (MOIN CC), Department of Radiology and Neuroradiology, University Medical Center Schleswig-Holstein (UKSH), Kiel University, Kiel, Germany, 2Laboratory of Fluid Dynamics and Technical Flows, Forschungs campus STIMULATE, University of Magdeburg, Magdeburg, Germany, 3Department of Medical Physics and Medicine, University of Wisconsin School of Medicine and Public Health, Madison, WI, United States, 4Department of Mechanical Engineering and Radiology, University of Wisconsin School of Medicine and Public Health, Madison, WI, United States, 5Department of Radiology and Neuroradiology, University Medical Center Schleswig-Holstein, Kiel, Germany
An intracranial aneurysm is a life-threatening disease. Vessel-wall enhancement on black-blood MRI was associated with inflammation and proposed as a marker for higher aneurysm rupture risk. However, slow blood flow can mimic wall enhancement. Here, we studied the effects of flow rates, spatial resolution, and motion-sensitized driven equilibrium (MSDE) on black-blood MRI using printed aneurysm models. A hyperintense signal was observed in the models and co-localized with a slow flow. MSDE and higher flow rates reduced the hyperintensities. Slow-flow phenomena contribute substantially to aneurysm enhancement, vary with MRI parameters, and should be considered in rupture assessment.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.