Power Pitch
Pitch: Radiomics & Multiparametric MR in Cancer
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

Power Pitch Session: How it Works
1st Hour: 2-minute Power Pitches in the Power Pitch Theater.
2nd Hour: 60-minute digital poster presentations at the smaller screens around the perimeter of the Power Pitch Theater.
| 09:15 | 0034.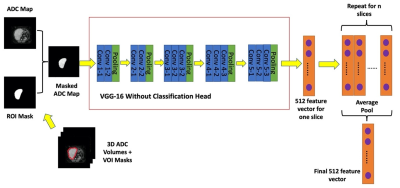 |
Test-retest repeatability of data-driven radiomic features derived from a deep-learning model: Diffusion-weighted MRI of soft-tissue sarcoma
Timothy Sum Hon Mun1,2, Imogen Thrussell1,2, Jessica Winfield1,2, Amani Arthur3, David J Collins1, Dow-Mu Koh1, Paul Huang3, Simon J Doran1, Christina Messiou1, and Matthew D Blackledge1
1Division of Radiotherapy and Imaging, Institute of Cancer Research, London, United Kingdom, 2Department of Radiology, Royal Marsden NHS Foundation Trust, London, United Kingdom, 3Institute of Cancer Research, London, United Kingdom
Monitoring treatment response of soft-tissue sarcomas (STS) following radiotherapy is challenging due to the inherent intratumoral heterogeneity of the disease. Radiomics and deep-learning provide opportunities for the discovery of potent biomarkers of treatment response. Successful response biomarkers must demonstrate good baseline repeatability if they are to be used for personalized treatment. We explore the stability of radiomic features derived from a deep-learning pipeline by determining the pairwise correlation of derived features, and measuring the baseline repeatability of features derived from the Apparent Diffusion-Coefficient maps. We demonstrate that 81/512 features are both independent and stable at repeat baseline measurement.
|
|
| 09:17 | 0035.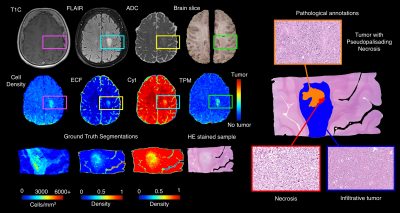 |
Radio-pathomic tumor probability maps in glioma patients using autopsy tissue samples as ground truth
Samuel Bobholz1, Allison Lowman2, Michael Brehler2, Savannah Duenweg1, John Sherman1, Fitzgerald Kyereme2, Elizabeth Cochran3, Dylan Coss3, Jennifer Connelly4, Wade Mueller5, Mohit Agarwal2, Anjishnu Banerjee6, and Peter LaViolette2,7
1Biophysics, Medical College of Wisconsin, Milwaukee, WI, United States, 2Radiology, Medical College of Wisconsin, Milwaukee, WI, United States, 3Pathology, Medical College of Wisconsin, Milwaukee, WI, United States, 4Neurology, Medical College of Wisconsin, Milwaukee, WI, United States, 5Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, United States, 6Biostatistics, Medical College of Wisconsin, Milwaukee, WI, United States, 7Biomedical Engineering, Medical College of Wisconsin, Milwaukee, WI, United States
This study used autopsy tissue samples to develop multi-stage radio-pathomic models of tumor probability in glioma patients. Three models were trained to predict cell density, extracellular fluid density, and cytoplasm density segmented from autopsy samples using T1, T1C, FLAIR, and ADC intensity. A fourth model was then trained to predict tumor probability from pathological annotations using the cellularity, extracellular fluid, and cytoplasm segmentations as input. The combined models were then able to non-invasively estimate tumor probability using MRI. These maps identified regions of tumor beyond the contrast-enhancing region and discriminated between areas of tumor and vasogenic edema within FLAIR hyperintensity.
|
|
| 09:19 | 0036.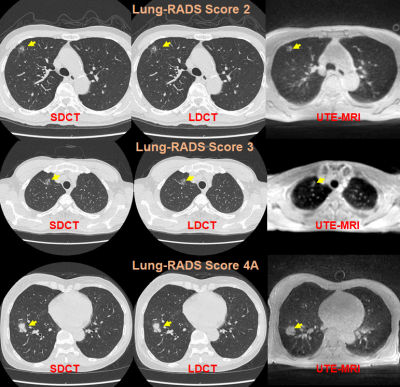 |
MRI with UTE: Capability for Nodule Detection and Lung RADS Classification as Compared with Standard- and Reduced-Dose CTs in Screening Cohort
Yoshiharu Ohno1,2, Masao Yui3, Kaori Yamamoto3, Daisuke Takenaka4, Takeshi Yoshikawa4, Masato Ikedo3, Saki Takeda5, Akiyoshi Iwase5, Yuka Oshima1, Nayu Hamabuchi1, Satomu Hanamatsu1, Yuki Obama1, Hiroyuki Nagata1, Takahiro Ueda1, Hirotaka Ikeda1, Kazuhiro Murayama2, and Hiroshi Toyama1
1Radiology, Fujita Health University School of Medicine, Toyoake, Japan, 2Joint Research Laboratory of Advanced Medical Imaging, Fujita Health University School of Medicine, Toyoake, Japan, 3Canon Medical Systems Corporation, Otawara, Japan, 4Diagnostic Radiology, Hyogo Cancer Center, Akashi, Japan, 5Radiology, Fujita Health University Hospital, Toyoake, Japan
No report has been found to compare nodule detection and Lung RADS classification capabilities in lung cancer screening cohort among pulmonary MR imaging with UTE, low-dose CT (LDCT) and standard-dose CT (SDCT). We hypothesized that pulmonary MR imaging with UTE has a similar potential to detect pulmonary nodules and evaluate Lung-RADS classification and can apply lung cancer screening as well as CT. The purpose of this study was to compare the capability for nodule detection and Lung RADS classification among pulmonary MR imaging with UTE, LDCT and SDCT in lung cancer screening population.
|
|
| 09:21 | 0037.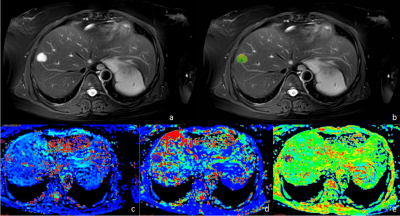 |
Differentiation between benign liver lesions, primary malignant and secondary malignant tumors using APT and IVIM imaging
Xue Ren1, Jiazheng Wang2, Liangjie Lin2, Qingwei Song1, Renwang Pu1, Ying Zhao1, Tao Lin1, Qihao Xu1, Zhiwei Shen2, and Ailian Liu1
1Department of Radiology, the First Affiliated Hospital of Dalian Medical University, Dalian, China, 2Philips Healthcare, Beijing, China
This study aims to assess the efficacy of amide proton transfer-weighted (APTw) combined with intravoxel incoherent motion (IVIM) imaging in differentiation benign liver lesions, primary malignant and secondary malignant tumors. Results showed that a high diagnostic efficacy could be achieved through the combination use of the APT value and IVIM parameters.
|
|
| 09:23 | 0038.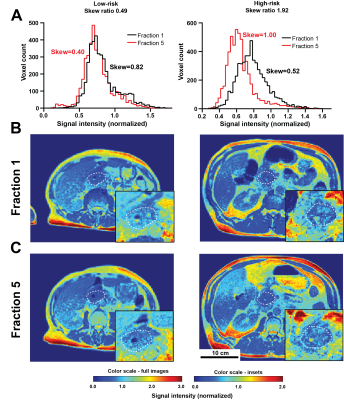 |
Delta radiomics analysis of Magnetic Resonance guided radiotherapy imaging data for treatment response prediction in pancreatic cancer
Michal R Tomaszewski1, Kujtim Latifi2, Emanuel Boyer3, Russell F Palm3, Issam El Naqa4, Eduardo G Moros2, Sarah E Hoffe3, Stephen A Rosenberg3, Jessica M Frakes3, and Robert J Gillies1
1Cancer Physiology, H Lee Moffitt Cancer Center, Tampa, FL, United States, 2Medical Physics, H Lee Moffitt Cancer Center, Tampa, FL, United States, 3Radiation Oncology, H Lee Moffitt Cancer Center, Tampa, FL, United States, 4Machine Learning, H Lee Moffitt Cancer Center, Tampa, FL, United States
Magnetic Resonance Image guided Stereotactic body radiotherapy (MRgRT) is increasingly used in treatment of multiple cancers including pancreatic adenocarcinoma (PDAC). We hypothesized that quantitative analysis (radiomics) of the longitudinal MRgRT imaging during treatment can help predict response. MRgRT TrueFISP images from n=26 non-resectable PDAC patients were analyzed and image feature ratios last/first fraction quantified. Image normalization to kidney signal was validated and robustness of features assessed. Histogram skewness change demonstrated significant association with Progression Free Survival. This result shows promise for future application of the novel integrated framework for processing and quantification of MRgRT data presented here first time.
|
|
| 09:25 | 0039.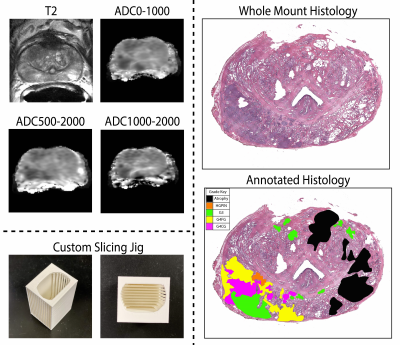 |
Radio-pathomic associations between MRI and complex histomorphometric features of prostate cancer
Savannah R. Duenweg1, Samuel A Bobholz2, Allison K Lowman3, Michael Brehler3, Fitzgerald Kyereme3, Kenneth A Iczkowski4, and Peter S LaViolette3,5
1Biophysics, Medical College of Wisconsin, Milwaukee, WI, United States, 2Biophysics, Medical College of Wisconsin, Wauwatosa, WI, United States, 3Radiology, Medical College of Wisconsin, Wauwatosa, WI, United States, 4Pathology, Medical College of Wisconsin, Wauwatosa, WI, United States, 5Biomedical Engineering, Medical College of Wisconsin, Wauwatosa, WI, United States
This study used digitized histology and MP-MRI from 48 patients with prostate cancer (PCa) to determine the relationship between complex histomorphometric features of PCa histology and MRI features. After prostatectomy, tissue samples were digitized, and co-registered to the T2 image. Slides were annotated by a pathologist, individual glands were identified using automated image processing algorithms, and histomorphometric features were calculated. Radiomic features were calculated to compare higher order texture and histological features. We found that lumen was more strongly associated with raw intensity, whereas epithelial features were more strongly associated with computed texture features.
|
|
| 09:27 | 0040.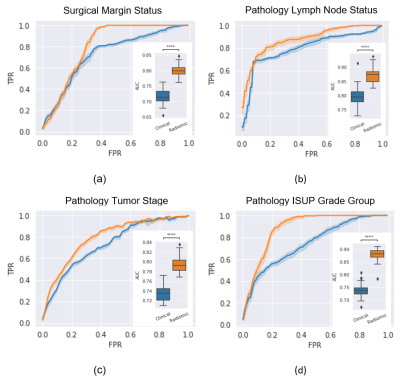 |
Predicting pathological status of prostate cancer patients – Examining the value and leading features in MRI Radiomics
Lars Johannes Isaksson1, Paul E Summers2, Matteo Johannes Pepa1, Mattia Zaffaroni1, Maria Giulia Vincini1, Giulia Corrao1,3, Giovanni Carlo Mazzola1,3, Marco Rotondi1,3, Sara Raimondi4, Sara Gandini4, Stefania Volpe1,3, Zaharudin Haron5, Sarah Alessi2, Paola Pricolo2, Francesco Alessandro Mistretta6, Stefano Luzzago6, Federico Cattani7, Gennaro Musi3,6, Ottavio De Cobelli3,6, Marta Cremonesi8,
Roberto Orecchia9, Giulia Marvaso1,3, Barbara Alicja Jereczek-Fossa1,3, and Giuseppe Petralia3,10
1Division of Radiation Oncology, IEO, European Institute of Oncology IRCCS, Milano, Italy, 2Division of Radiology, IEO, European Institute of Oncology IRCCS, Milano, Italy, 3Department of Oncology and Hemato-oncology, University of Milan, Milano, Italy, 4Department of Experimental Oncology, IEO, European Institute of Oncology IRCCS, Milano, Italy, 5Radiology Department, National Cancer Institute, Putrajaya, Malaysia, 6Division of Urology, IEO, European Institute of Oncology IRCCS, Milano, Italy, 7Unit of Medical Physics, IEO, European Institute of Oncology IRCCS, Milano, Italy, 8Radiation Research Unit, IEO, European Institute of Oncology IRCCS, Milano, Italy, 9Scientific Directorate, IEO, European Institute of Oncology IRCCS, Milano, Italy, 10Precision Imaging and Research Unit, IEO, European Institute of Oncology IRCCS, Milano, Italy
The risk of patients being under- or overtreated during radiotherapy depends heavily on the pre-treatment assessment. Prediction models for surgical margin status, pathological lymph nodes, pathological tumor stage and ISUP grade group were formed using clinical and radiological features alone and together with whole-prostate radiomic features in 100 patients who proceeded to prostatectomy after multiparametric-MRI. The addition of radiomics features significantly improved AUC for the prediction models. The leading radiomic features differed between the different models.
|
|
| 09:29 | 0041.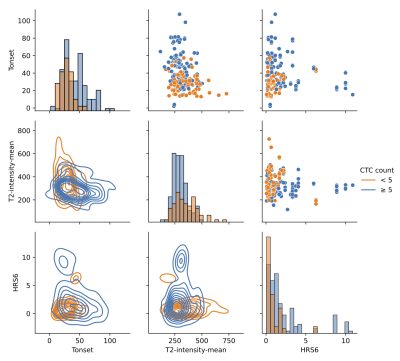 |
Radiomic Features Measured with Multiparametric Magnetic Resonance Imaging Predict Prostate Cancer Metastatic Risk
Mohammad Alhusseini1, Adrian L Breto1, Isaac L Xu1, Ahmad Algohari1, Sandra M Gaston1, Matthew C Abramowitz1, Alan Dal Para1, Sanoj Punnen2, Alan Pollack1, and Radka Stoyanova1
1Department of Radiation Oncology, University of Miami Miller School of Medicine, Miami, FL, United States, 2Department of Urology, University of Miami Miller School of Medicine, Miami, FL, United States
Circulating tumor cells (CTCs) have been shown to be an indicator for metastatic risk in prostate cancer. We investigated the association between radiomics features extracted from multiparametric MRI of the prostate and CTC counts in prostate cancer patients enrolled in two institutional clinical trials (n=71). We trained a neural network to predict the dichotomized CTCs counts, defined by a 5 CTCs threshold. The top seven features, ranked using maximum-relevance minimum-redundancy, were used as input to a neural network. The training and testing were repeated for 100 runs of 5-Fold cross validation, resulting in AUC 0.834 to predict CTCs ≥ 5.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.