Digital Poster
Motion Correction I
ISMRM & ISMRT Annual Meeting & Exhibition • 03-08 June 2023 • Toronto, ON, Canada

| Computer # | |||
|---|---|---|---|
1818.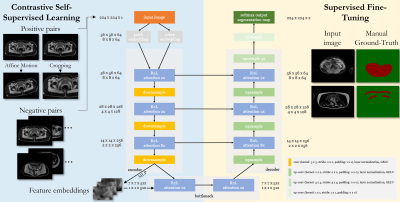 |
1 | Self-supervised contrastive learning for motion artifact detection in whole-body MRI: Quality assessment across multiple cohorts
Thomas Küstner1, Jan Borst1, Dominik Nickel2, Fabian Bamberg3, Marcel Früh1, and Sergios Gatidis1,4
1Medical Image and Data Analysis (MIDAS.lab), Department of Diagnostic and Interventional Radiology, University Hospital of Tuebingen, Tuebingen, Germany, 2Siemens Healthineers, Erlangen, Germany, 3Department of Diagnostic and Interventional Radiology, University of Freiburg, Freiburg, Germany, 4Max Planck Institute for Intelligent Systems, Tuebingen, Germany Keywords: Machine Learning/Artificial Intelligence, Motion Correction, Motion Detection, Self-Supervised Learning Motion is still one of the major extrinsic sources for imaging artifacts in MRI that can strongly deteriorate image quality. Any impairment by motion artifacts can reduce the reliability and precision of the diagnosis and a motion‐free reacquisition can become time‐ and cost‐intensive. Furthermore, in large-scale epidemiological cohorts, manual quality screening becomes impracticable. An automated quality assessment is thus of interest. Reliable motion estimation in varying domains (imaging sequences, multiple scanners, sites) is however challenging. In this work, we propose an attention-based transformer that can detect motion in various MR imaging scenarios. |
|
1819.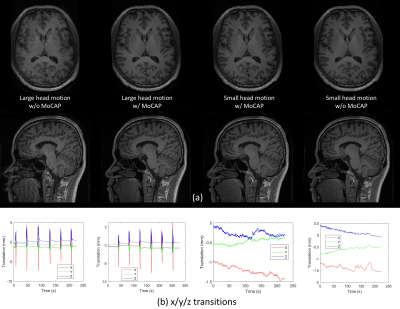 |
2 | Effectiveness and Reliability Assessment on Head Motion Capturing and Correction (MoCAP)
Zhuoyang Gu1,2, Lianghu Guo1, Qing Yang1, Xinyi Cai1, Tianli Tao1, Sifan He1, Hua Jiang1, Haifeng Tang1, Qian Wang1, Xiaopeng Zong1, Dinggang Shen1, Qiang He2, and Han Zhang1
1School of Biomedical Engineering, ShanghaiTech University, Shanghai, China, 2United Imaging Healthcare Co., Ltd., Shanghai, China Keywords: Motion Correction, Brain, MRI acquisition Head motion monitoring and compensation during MRI is essential to imaging quality and success rates of acquisition. MoCAP is a novel real-time head motion monitoring and correction technique utilizing structured light to perform prospective motion correction by adjusting MR gradient. We perform effectiveness and reliability assessment on MoCAP in structural and functional MRI. MoCAP can significantly improve quality of the two modalities, reducing motion artifacts and enhancing validity and reliability of post-processing results. MoCAP is promising in the MRI field for special populations like children and patients with difficulty in keeping still during scan. |
|
1820.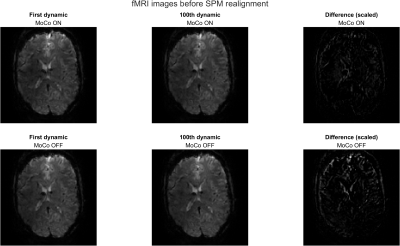 |
3 | Prospective Motion Correction for 3D-EPI fMRI using Orbital Navigators and Linear Regression
Thomas Ulrich1, Malte Riedel1, and Klaas Prüssmann1
1Institute for Biomedical Engineering, ETH Zurich and University of Zurich, Zürich, Switzerland Keywords: Motion Correction, fMRI (resting state) We propose to apply a navigator-based prospective motion correction method to 3D-EPI fMRI scans. The method is investigated during in-vivo volunteer experiments with and without intentional motion. Post-processing with SPM12 shows that head motion is largely compensated by the navigator method. We show that the technique is sensitive to breathing motion and cardioballistic motion by comparing to physiological measurements from a breathing belt and pulse oximeter, and we show that it strongly reduces inter-volume displacements. |
|
1821.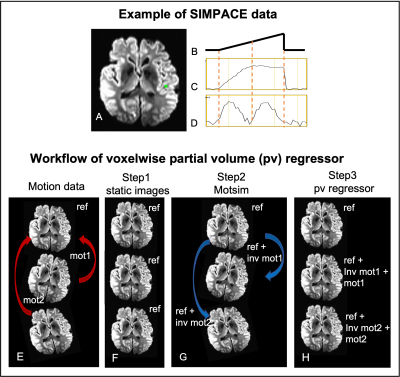 |
4 | Effective removal of the residual head motion artifact after motion correction in fMRI data.
Wanyong Shin1 and Mark J Lowe1
1Radiology, Cleveland Clinic, Cleveland, OH, United States Keywords: Motion Correction, Brain, Head motion, fMRI We investigate the source of residual motion artifact after volumetric motion correction using a custom MR sequence acquisition with prospectively injected motion (SIMPACE). We injected various patterns of motion during scanning ex-vivo brain phantoms at 3T to synthesize head motion in an fMRI dataset. We propose voxelwise retrospective motion regressors in addition to 6 rigid body motion parameters, then compare it with the “standard” 6 motion parameters and their derivatives regressor models. The proposed model improves tSNR by 40% and 11% in linear drift of motion and the realistic motion case, respectively, compared to 6 motion parameter model. |
|
1822.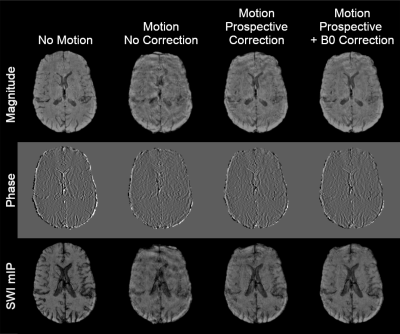 |
5 | Combined motion and B0 correction of susceptibility weighted imaging with jointly acquired FID and spherical navigators
Miriam Hewlett1,2, Junmin Liu2, and Maria Drangova1,2
1Medical Biophysics, Western University, London, ON, Canada, 2Robarts Research Institute, London, ON, Canada Keywords: Motion Correction, Motion Correction Jointly acquired FID and spherical navigators were applied for prospective correction of motion and retrospective correction of zeroth order field offsets in a susceptibility weighted imaging protocol. Initial results showed a significant reduction in motion artifacts with prospective motion correction, quantified using structural similarity index. Retrospective B0 correction provided an additional improvement in image quality (also statistically significant). Future work will investigate methods to reduce residual artifacts, as well as incorporating the navigator within deadtime in the sequence to reduce scan time. |
|
1823.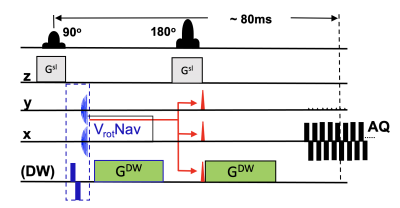 |
6 | Velocity-Encoding Navigator for First-Order Motion Compensation in Diffusion MRI
Bo Li1 and Thomas Ernst1
1Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland, Baltimore, Baltimore, MD, United States Keywords: Motion Correction, Diffusion/other diffusion imaging techniques, velocity navigator Uncorrected head rotations during diffusion weighted imaging (DWI) can induce gradient imbalances that may shift the echo outside of the k-space window and cause signal dropouts. Therefore, we developed a rotational velocity navigator (~10ms duration) that is acquired immediately after each excitation and estimates the rotational velocities perpendicular to the diffusion gradient. The accuracy of estimated velocities was 4.1°/s relative to an optical tracking system (“gold standard”). Ultimately, the rotational velocities can predict the gradient moment error in first order, and a gradient blip can be applied to recenter the echo in k-space. |
|
1824.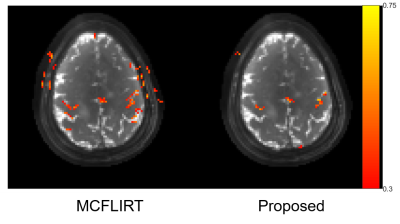 |
7 | Fast, model-based, and navigator-free retrospective motion correction for non-Cartesian fMRI
Guanhua Wang1, Shouchang Guo2, Jeffrey A. Fessler2, and Douglas C. Noll1
1Biomedical Engineering, University of Michigan, Ann Arbor, MI, United States, 2EECS, University of Michigan, Ann Arbor, MI, United States Keywords: Motion Correction, Motion Correction This abstract presents a retrospective motion correction method for fMRI. The method alternates between motion estimation and motion-informed model-based reconstruction. Compared to registration-based correction, this approach resolves intra-frame, inter-shot motion without additional navigators. The open-source GPU-based implementation enables efficient correction/reconstruction for large-scale non-Cartesian fMRI data. With prospective experiments, we demonstrate that our approach outperformed retrospective registration by providing higher-resolution images with reduced false positives in activation maps. |
|
1825.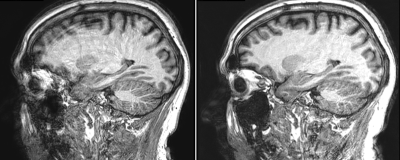 |
8 | Inline Retrospective Motion Correction and Dynamical Alignment Using an Optical Markerless Motion Tracker
Ulrich Lindberg1, Stefan Glimberg2, Gerard Crelier3, Martin Buehrer3, and Henrik Bo Wiberg Larsson1
1Functional Imaging Unit, Department of Clinical Physiology and Nuclear Medicine, Rigshospitalet, Copenhagen, Denmark, 2TracInnovations, Ballerup, Denmark, 3GyroTools, Zurich, Switzerland Keywords: Image Reconstruction, Motion Correction Using an external motion tracking system for recording head movement during MR-acquisition and a modified version of the Philips reconstruction software (Recon2.0) that enables phase re-alignment and re-gridding of acquisition profiles during the standard immediate reconstruction process, we present a fully integrated retrospective motion correction solution that deliver both corrected and non-corrected images to the user and allows for the immediate visual assessment of the correction quality upon acquisition. |
|
1826.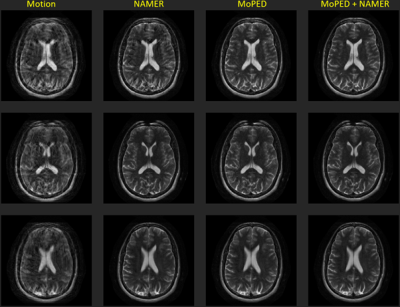 |
9 | Severe MR Motion Artefact Correction with 2 step Deep Learning-based guidance
Julian Hossbach1,2, Daniel Nicolas Splitthoff2, Bryan Clifford3, Daniel Polak4,5, Stephan Cauley5, and Andreas Maier1
1Pattern Recognition Lab, Friedrich-Alexander-University Erlangen-Nuremberg, Erlangen, Germany, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Siemens Medical Solutions, Boston, MA, United States, 4Siemens Healthcare Gmbh, Erlangen, Germany, 5Department of Radiology, Athinoula A. Martinos Center for Biomedical Imaging, Boston, MA, United States Keywords: Machine Learning/Artificial Intelligence, Motion Correction Motion artifacts can pose a difficult challenge in the clinical workflow. For addressing this issue, we here investigate the performance of two Deep Learning based motion mitigation strategies, MoPED and NAMER, and demonstrate that both approaches can readily be combined. This allows for the correction of severely corrupted images. |
|
1827.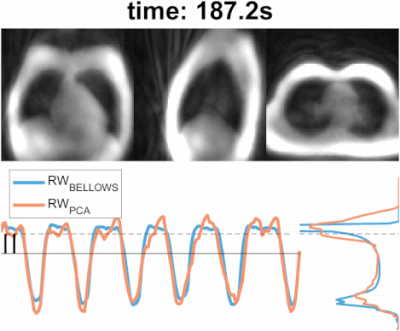 |
10 | Self-navigated free-breathing ZTE lung imaging
Jose de Arcos1, Ana Beatriz Solana2, Jonathan Weir-McCall3,4, Emil Ljungberg5,6, Joshua D Kaggie3, and Florian Wiesinger2
1GE HealthCare, Little Chalfont, Amersham, United Kingdom, 2ASL Europe, GE HealthCare, Munich, Germany, 3Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 4University of Cambridge School of Clinical Medicine, Royal Papworth Hospital, Cambridge, United Kingdom, 5Medical Radiation Physics, Lund University, Lund, Sweden, 6Neuroimaging, Institute of Psychiatry, Psychology, and Neuroscience, King's College London, London, United Kingdom Keywords: Motion Correction, Motion Correction, Self-navigation Here we propose a self-navigated technique, based on the extraction of respiratory motion estimates using interleaved spiral phyllotaxis trajectories in 3D radial zero echo time (ZTE) acquisitions. These are particularly well-suited to capture the short T2* signal, characteristic of lung parenchyma, and have high acquisition efficiency allowing to generate fast temporal resolution navigators. The self-navigation technique worked robustly for different respiratory patterns and on 1.5T and 3.0T fields strengths, obtaining high quality images comparable to the ones obtained with bellows-gating. |
|
1828.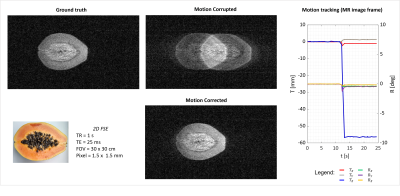 |
11 | Optical camera calibration to implement various marker-based motion correction techniques in open geometry 0.5 T upright scanner
Laura Bortolotti1, Olivier Mougin1, Paul Glover1, Richard Bowtell1, and Penny Gowland1
1SPMIC, University of Nottingham, Nottingham, United Kingdom Keywords: Motion Correction, Motion Correction, Marker-based, calibration Open MRI scanner improves patient comfort but allows movement during scanning. Hence, implementation of MoCo techniques is crucial to maximize image quality. Here, we established a method to use a standard commercial optical motion tracking set-up in an Open MRI scanner. The optical system has been calibrated to provide tracking measurements in the image reference frame. Then, a simple phantom movement was tested and corrected using NUFFT algorithm in BART. |
|
1829.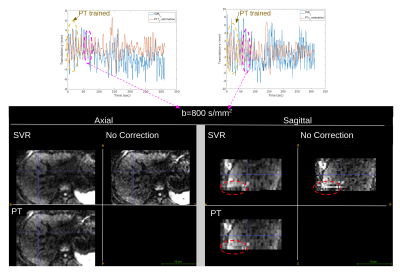 |
12 | Pilot Tone-navigated motion estimation for liver DW-MRI
Cemre Ariyurek1, Serge Vasylechko1, Xiaodong Zhong2, Vibhas Deshpande3, Michael Bush4, Stephan Voss1, Onur Afacan1, and Sila Kurugol1
1Radiology, Boston Children's Hospital and Harvard Medical School, Boston, MA, United States, 2MR R&D Collaborations, Siemens Medical Solutions USA, Inc., Los Angeles, CA, United States, 3MR R&D Collaborations, Siemens Medical Solutions USA, Inc., Austin, TX, United States, 4MR R&D Collaborations, Siemens Medical Solutions USA, Inc., New York, NY, United States Keywords: Motion Correction, Diffusion/other diffusion imaging techniques Diffusion-weighted MRI (DW-MRI) is capable of detecting and characterizing liver tumors and following-up treatments. Unfortunately, respiratory motion during DW-MRI scan causes misalignments between slices and reduces image quality. 3D slice-to-volume registration (SVR) can be employed to correct for motion. However, motion estimates may be inaccurate for high b-value images where SNR decreases. In this work, we propose to use PT estimated motion correction, which is calibrated on 3D SVR motion parameters obtained from low b-value images. We showed misalignments between slices are reduced by the proposed PT-based motion correction compared to SVR-based motion correction and no correction. |
|
1830.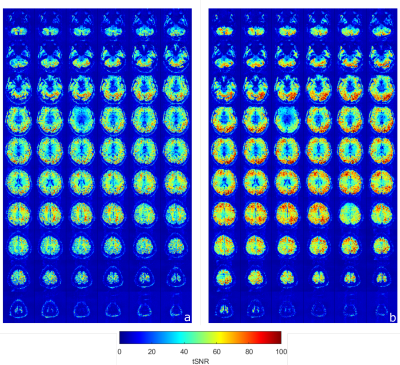 |
13 | Multislice-to-volume Prospective Motion Correction for Functional MRI Protocols at 7T
Steven Winata1, Daniel Christopher Hoinkiss2, Graeme Alexander Keith1, Salim Mohammed al-Wasity1, and David Andrew Porter1
1Imaging Centre of Excellence, University of Glasgow, Glasgow, Scotland, 2Fraunhofer Institute for Digital Medicine MEVIS, Bremen, Germany Keywords: Motion Correction, fMRI, Prospective motion correction; Markerless; Real-time 7 Tesla MRI provides higher signal-to-noise ratio (SNR) and spatial resolution, but is also more sensitive to motion-induced artefacts. The restricted environment within 7T scanners make non-hardware options for motion correction attractive. In this abstract, we investigate the use of the markerless, image-based, real-time Multislice Prospective Acquisition Correction (MS-PACE) technique for functional MRI protocols at 7T. This multislice scheme allows for a sub-TR motion detection and correction. It is demonstrated that the technique is able to correct longer-term motion components occurring during the acquisition. |
|
1831.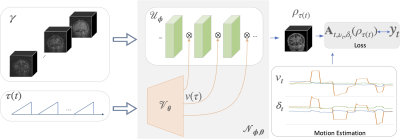 |
14 | Motion compensated multi-contrast MRI using deep factor model
Yan Chen1, James H. Holmes1, Curtis A. Corum2, Vincent Magnotta1, and Mathews Jacob1
1University of Iowa, Iowa City, IA, United States, 2Champaign Imaging, LLC, Minneapolis, MN, United States Keywords: Motion Correction, Multi-Contrast Recent quantitative parameter mapping methods including MR fingerprinting collect a time series of images that capture the evolution of magnetization. The focus of this work is to introduce a novel approach termed as deep factor model, which offers an efficient representation of the multi-contrast image time series. The higher efficiency of the representation enables the acquisition of the images in a highly undersampled fashion, which translates to reduced scan time in 3D high-resolution multi-contrast applications. The approach integrates motion estimation and compensation, making the approach robust to subject motion during the scan. |
|
1832.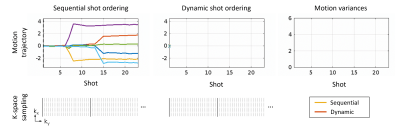 |
15 | A Dynamic 2D TSE Acquisition Strategy for Robust SAMER Motion Mitigation
Daniel Polak1, Daniel Nicolas Splitthoff1, Bryan Clifford2, Wei-Ching Lo2, Yantu Huang3, Susie Huang4, John Conklin4, Lawrence L. Wald5, and Stephen F. Cauley5
1Siemens Healthcare GmbH, Erlangen, Germany, 2Siemens Medical Solutions, Boston, MA, United States, 3Shenzhen Magnetic Resonance, Shenzhen, China, 4Massachusetts General Hospital, Boston, MA, United States, 5A. A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States Keywords: Motion Correction, Brain, Value, Clinical Application Retrospective motion correction for 2D TSE/FSE is challenging due to interpolation through slices with gaps, interleaved slice orderings, and spin history effects. Optimized Cartesian sampling trajectories provide decreased motion sensitivity in specific situations but can also exacerbate motion sensitivity under typical patient motion. In this work, we introduce a dynamic acquisition strategy that determines the k-space lines acquired in the next shot based on the prior patient motion. Specifically, each TR dynamically encodes available lines which minimize motion variance. We show that this dynamic acquisition strategy results in improved reconstruction robustness under typical clinical motion scenarios. |
|
1833.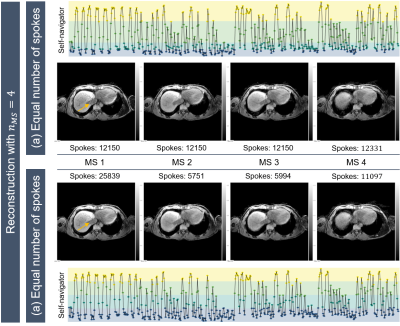 |
16 | Patient-specific respiratory liver motion analysis for individual motion-resolved reconstruction
Veronika J Spieker1,2, Jonathan K Stelter3, Veronika A Zimmer2, Kilian Weiss4, Rickmer F Braren3, Dimitrios Karampinos3, and Julia A Schnabel1,2,5
1Helmholtz Center Munich, Munich, Germany, 2School of Computation, Information and Technology, Technical University of Munich, Munich, Germany, 3Department of Diagnostic and Interventional Radiology, Technical University of Munich, Munich, Germany, 4Philips GmbH Market DACH, Hamburg, Germany, 5School of Biomedical Imaging and Imaging Sciences, King's College, London, United Kingdom Keywords: Motion Correction, Liver Respiratory motion is a major source of artefacts in abdominal MRI and varies considerably across subjects. Motion-resolved strategies utilize the periodic nature of respiratory motion, but do not always consider absolute patient-specific motion information. The purpose of the present work is to develop a framework incorporating the patient-specific absolute body motion into a motion-resolved reconstruction (XD-GRASP), while adapting different motion binning strategies. To explore the potential of respiratory motion knowledge for MR reconstruction, individual absolute body motion, i.e. of the liver, is identified. |
|
1834.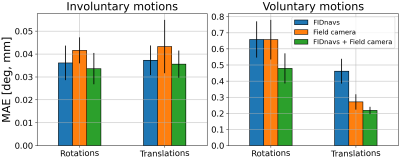 |
17 | Combining FID navigators with field probe monitoring for improved head motion tracking and prospective correction at 7T
Matthias Serger1, Ruediger Stirnberg1, Philipp Ehses1, and Tony Stoecker1,2
1German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 2Department of Physics and Astronomy, University of Bonn, Bonn, Germany Keywords: Motion Correction, Motion Correction FID navigators and field probes can measure local motion-induced magnetic field changes at different positions. Both methods are suited for marker-less prospective motion correction but with limited accuracy. A small 7T study with five subjects was performed to compare their performance for small involuntary and large voluntary motion. It is demonstrated that errors of regression models, calibrated between magnetic field measurements and motion parameters, are consistently reduced by combining both techniques. Furthermore, a proof-of-concept prospective motion correction experiment based on FID navigators is presented, which will be extended to include field probe measurements in the near future. |
|
1835.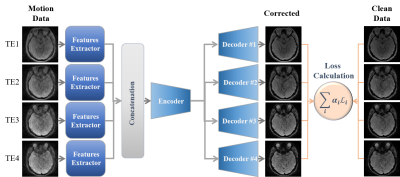 |
18 | Multi-Echo MRI Motion Artifact Reduction via Knowledge Interaction Learning for Better SWI Enhancement
Mohammed A. Al-masni1, Seul Lee2, Sewook Kim2, Sung-Min Gho3, Young Hun Choi4, and Dong-Hyun Kim2
1Department of Artificial Intelligence, Sejong University, Seoul, Korea, Republic of, 2Department of Electrical and Electronic Engineering, Yonsei University, Seoul, Korea, Republic of, 3GE Healthcare, Korea, Seoul, Korea, Republic of, 4Department of Radiology, Seoul National University Hospital, Seoul, Korea, Republic of Keywords: Motion Correction, Artifacts Patient movement during MRI scan can cause severe degradation of image quality. In Susceptibility-Weighted Imaging (SWI), several echoes are measured during a single repetition period, where the earliest echoes show less contrast between various tissues, while the higher echoes are more susceptible to artifacts and signal dropout. This paper proposes a data-driven retrospective deep learning method by taking the advantage of interactively learning multiple echoes together through sharing their knowledge using unified training parameters. The proposed method allows to share information and gain an understanding of the correlations between multiple echoes towards generating high-resolution susceptibility enhanced contrast images. |
|
1836.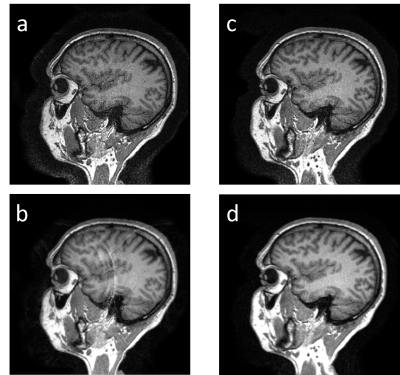 |
19 | Highly Accelerated 3D MPRAGE using Robust SAMER Motion Mitigation
Daniel Nicolas Splitthoff1, Stephen Cauley2, Tobias Kober3, Julian Hossbach1, Bryan Clifford4, Wei-Ching Lo4, Yan Tu Huang5, Susie Y. Huang6, John Conklin6, Lawrence L. Wald2, and Daniel Polak1
1Siemens Healthcare GmbH, Erlangen, Germany, 2A. A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 3Siemens Healthineers International AG, Lausanne, Switzerland, 4Siemens Medical Solutions, Boston, MA, United States, 5Shenzhen Magnetic Resonance Ltd.,, Shenzhen, China, 6Massachusetts General Hospital, Charlestown, MA, United States Keywords: Motion Correction, Data Acquisition, Fast imaging, Compressed Sensing Long clinical acquisitions can face the challenge of motion during the scan. One approach to address this problem is the recently introduced SAMER retrospective motion correction technique for 3D MPRAGE. As an alternative, it has been suggested to shorten the scan time by acceleration techniques, e.g., using Compressed Sensing, and thus reduce the likelihood of patient discomfort and severe motion. We here combine SAMER with Compressed Sensing for high acceleration factors (R=6). |
|
1837.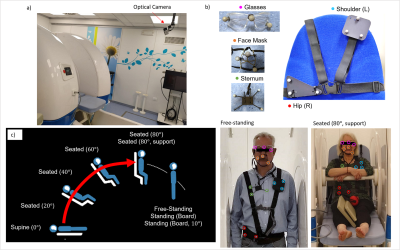 |
20 | Evaluation of body motion at various patient position in a 0.5 T Upright scanner
Laura Bortolotti1, Isabel Clennell 1, Amy Bradbury1, Olivier Mougin1, Paul Glover1, Richard Bowtell1, and Penny Gowland1
1SPMIC, University of Nottingham, Nottingham, United Kingdom Keywords: Motion Correction, Motion Correction, Marker based tracking Body motion at nine typical upright MRI poses (standing, seated, supine), have been characterised for several body positions (head, shoulder, sternum, hip) for 20 subjects over 30 s (free breathing); and 20 s (breath hold) and for 9 subjects over 10 minutes. Free-standing caused most motion; lying supine caused least. The use of a body support and breath-holding reduced motion. Net motion was similar over 50-1000 ms sampling periods. Respiratory-related motion could be removed from tracking data. The degree of motion identified in this work will inform future respiratory triggering and motion correction work. |
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.