Oral Session
Motion Detection & Correction Techniques
ISMRM & ISMRT Annual Meeting & Exhibition • 03-08 June 2023 • Toronto, ON, Canada

15:45 |
1009.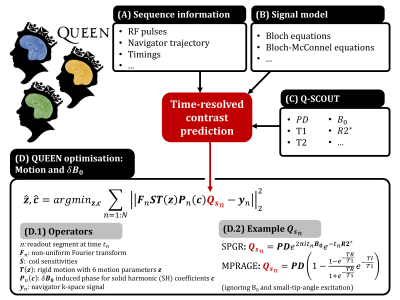 |
Towards rapid and accurate navigators for motion and B0
estimation using QUEEN (QUantitatively-Enhanced parameter
Estimation from Navigators)
Yannick Brackenier1,
Nan Wang1,
Congyu Liao1,
Xiaozhi Cao1,
Sophie Schauman1,
Mahmut Yurt2,
Lucilio Cordero-Grande3,
Shaihan J Malik4,5,
Adam Kerr2,6,
Joseph V Hajnal4,5,
and Kawin Setsompop1,2
1Department of Radiology, Stanford University, Stanford, CA, United States, 2Department of Electrical Engineering, Stanford University, Stanford, CA, United States, 3Biomedical Image Technologies, ETSI Telecomunicación, Universidad Politécnica de Madrid and CIBER-BNN, Madrid, Spain, 4Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 5Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 6Cognitive and Neurobiological Imaging (CNI), Stanford University, Stanford, CA, United States Keywords: Motion Correction, Brain ‘Scout-based’ navigators exploit correlations between navigator data and a low-resolution multi-coil pre-scan data (scout) to effectively estimate either motion or B0-perturbations. Usually, scout data has a fixed contrast, limiting their usage in estimating motion within echo-trains where contrast changes from one readout to the next (e.g. MPRAGE). Furthermore, combined motion and B0-perturbation estimation from rapid navigators has yet to be achieved. In this work, we propose a quantitative scout (Q-SCOUT) to ‘time-resolve’ navigator contrast, along with a rapid SPINS-navigator (few ms). Q-SCOUT and rapid navigator data are used in our QUEEN method to enable within-echo-train motion and B0-perturbation estimation. |
| 15:53 |
1010.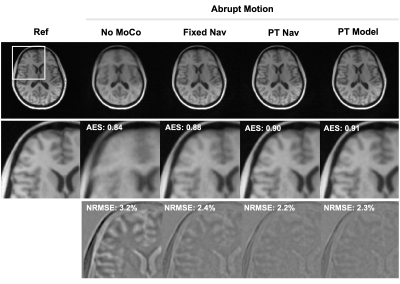 |
High Temporal Resolution Head Motion Tracking using Pilot Tone
and 3D Radials
Tess E Wallace1,2,
Cemre Ariyurek1,2,
Fatih Calakli1,2,
Tobias Kober3,4,5,
Simon K Warfield1,2,
and Onur Afacan1,2
1Computational Radiology Laboratory, Boston Children's Hospital, Boston, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3Advanced Clinical Imaging Technology, Siemens Healthineers International AG, Lausanne, Switzerland, 4Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 5LTS5, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland Keywords: Motion Correction, Brain Radial acquisitions are inherently motion-robust and facilitate self-navigation, however the frequency of motion updates from navigator images is limited. Pilot tone (PT) enables continuous motion sensing, but estimation of quantitative motion parameters requires a subject-specific calibration. In this work, we propose (i) using PT motion detection to guide navigator-based motion estimation from a 3D radial acquisition and (ii) using these measurements to calibrate a PT motion model in order to provide high temporal resolution quantitative motion tracking. This hybrid approach demonstrates improved retrospective correction results with reduced blurring and facilitates PT motion tracking for subsequent scans. |
| 16:01 |
1011.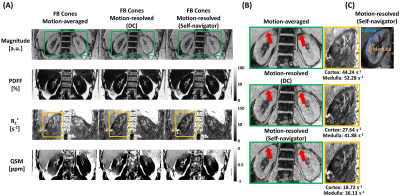 |
Free-breathing renal R2*/QSM using 3D multi-echo UTE cones
acquisition and motion-resolved reconstruction with image-based
self-navigator
MungSoo Kang1,
Gerald G. Behr2,
Ilya Glezerman3,
Ricardo Otazo1,2,
and Youngwook Kee1
1Department of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 3Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, United States Keywords: Motion Correction, Image Reconstruction Respiratory motion is a significant challenge for the acquisition of reliable renal R2*/QSM. The combination of 3D multi-echo UTE cones MRI and respiratory motion-resolved image reconstruction with an image-based self-navigator was developed to enable motion-robust renal R2*/QSM. Motion-resolved reconstruction with an image-based self-navigator showed better image quality compared to conventional gridding reconstruction and higher apparent SNR and CNR compared to motion-resolved reconstruction using the center of the k-space as navigator. 3D multi-echo UTE cones MRI and motion-resolved image reconstruction with image-based self-navigator demonstrated the feasibility of motion-robust, free-breathing, and high isotropic resolution of renal R2*/QSM. |
| 16:09 |
1012.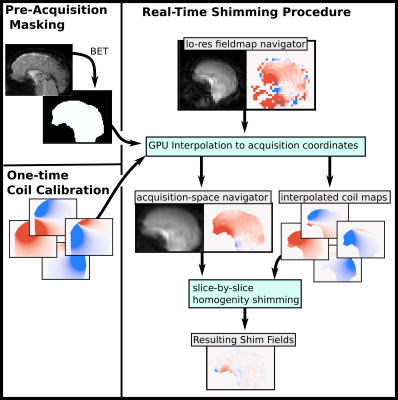 |
Motion-Compensated Slice-by-Slice ∆B0 Shimming with an AC/DC
Shim Coil and Dual-Echo vNavs
Nicolas Sebastian Arango1,
Robert Frost2,3,
Paul Wighton2,
Jason Stockmann2,3,
Ovidiu C Andronesi2,3,
and Andre van der Kouwe2,3
1Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 2A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 3Department of Radiology, Harvard Medical School, Boston, MA, United States Keywords: Motion Correction, fMRI Subject motion results in intra-scan ∆B0 changes which are uncompensated in conventional ∆B0 shimming methods. Rapidly switchable shim currents and ∆B0 vNavigators together enable motion-compensated shimming. We have demonstrated successful measurement, calculation, and application of motion-compensated slice-by-slice shims using and AC/DC coil and vNav ∆B0 maps. Motion-compensated slice-by-slice homogeneity shimming improves shim robustness to subject motion and enables compatibility with changing slice prescriptions of prospective motion correction. |
| 16:17 |
1013.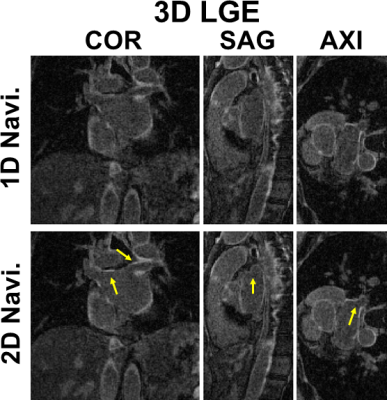 |
2D Self-Navigation Improves Respiratory Motion Tracking Compared
with 1D Self-Navigation in 3D Left Atrial LGE using XD-GRASP
Reconstruction
KyungPyo Hong1,
Suvai Gunasekaran1,
Mohammed Elbaz1,
Aggelos K Katsaggelos2,
Saman Nazarian3,
Rod Passman4,
Eugene Kholmovski5,
and Daniel Kim1
1Radiology, Northwestern University Feinberg School of Medicine, Chicago, IL, United States, 2Electrical and Computer Engineering, Northwestern University Feinberg School of Medicine, Evanston, IL, United States, 3Medicine, Cardiology, Hospital of the University of Pennsylvania, Philadelphia, PA, United States, 4Medicine, Cardiology, Northwestern University Feinberg School of Medicine, Chicago, IL, United States, 5Biomedical Engineering, Johns Hopkins University, Baltimore, MD, United States Keywords: Motion Correction, Arrhythmia, Late Gadolinium Enhancement Previously described left atrial (LA) late gadolinium enhancement (LGE) using a stack-of-stars k-space sampling pattern with XD-GRASP reconstruction may produce blurry LA wall due to signal variation of inversion-recovery-prepared 1D self-navigation caused by arrhythmia. We hypothesize 2D image self-navigation would be less sensitive to arrhythmia for motion tracking in XD-GRASP framework. In this study, we developed a free-breathing, LA LGE pulse sequence with isotropic spatial resolution using a stack-of-stars sampling pattern and 2D self-navigation and compared its performance against conventional 1D self-navigation in patients with atrial fibrillation. Results show that 2D self-navigation improves respiratory motion tracking compared with 1D. |
| 16:25 |
1014.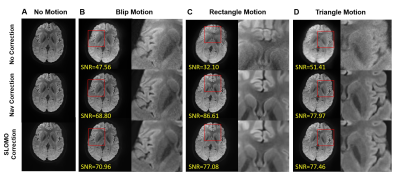 |
Motion Correction for Interleaved EPI Diffusion Imaging using a
Markerless Optical Tracking System
Yi Xiao1,
Chunyao Wang1,
Sisi Li1,
Huijun Chen1,
and Hua Guo1
1Center for Biomedical Imaging Research, Department of Biomedical Engineering, Tsinghua University, Beijing, China Keywords: Motion Correction, Diffusion Tensor Imaging Multi-shot interleaved EPI can achieve high-resolution diffusion imaging and effectively reduce geometric distortions. However, multi-shot acquisitions are susceptible to bulk motion which causes artifacts. Optical tracking-based motion correction is an effective method to reduce motion artifacts in DWI. Based on a self-developed Structured Light Optical MOtion tracking (SLOMO) system, a retrospective motion correction method was developed for multi-shot interleaved EPI DWI. The performance was evaluated by in-vivo experiments and compared with software-based correction. |
| 16:33 |
1015.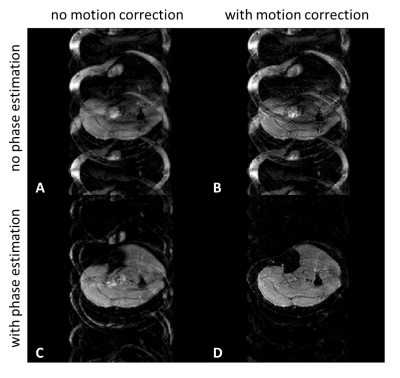 |
Self-navigation using fat navigators for motion immune diffusion
weighted-imaging via chemical-shift encoded multi-shot EPI
Yiming Dong1,
Kirsten Koolstra2,
Malte Riedel3,
Matthias J.P. van Osch1,
and Peter Börnert1,4
1C.J. Gorter MRI Center, Department of Radiology, LUMC, Leiden, Netherlands, 2Philips, Best, Netherlands, 3University and ETH Zurich, Zurich, Switzerland, 4Philips Research, Hamburg, Germany Keywords: Motion Correction, Motion Correction The presence of fat is a challenge in diffusion-weighted EPI. Recently, chemical-shift encoded approaches found interest, as a smart way of signal averaging, doing water/fat separation and diffusion phase navigation in the reconstruction. However, the dominant signal character of fat in diffusion could actually also be exploited as an advantage by forming a shot-specific fat navigator to track and correct for macroscopic in-plane motion, combined with a model-based self-navigated water/fat decomposition. This allows to correct for both physiological and macroscopic in-plane motion effects in DWI when estimating water and fat resolved images from chemical-shift encoded multi-shot EPI data. |
| 16:41 |
1016.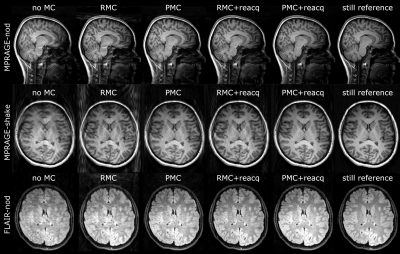 |
Selective MRI reacquisition to extend the working range of
retrospective motion correction
Malte Laustsen1,2,
Jakob Slipsager2,
Thomas Gaaß2,
Robert Frost3,4,
André van der Kouwe3,4,
Melanie Ganz5,6,
and Lars G. Hanson1
1Magnetic Resonance Section, DTU Health Tech, Technical University of Denmark, Kgs. Lyngby, Denmark, 2TracInnovations, Ballerup, Denmark, 3Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 4Department of Radiology, Harvard Medical School, Boston, MA, United States, 5Neurobiology Research Unit, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark, 6Department of Computer Science, University of Copenhagen, Copenhagen, Denmark Keywords: Motion Correction, Artifacts Retrospective motion correction (RMC) can substantially reduce motion artifacts in 3D brain MRI. However, for extensive motion, RMC performance is limited. We evaluate RMC with selective reacquisition (RMC+reacq) to expand the range of correctable motion, while directly comparing to prospective motion correction (PMC) for volumetric brain MRI, using external motion tracking. Both approaches lead to significant image quality improvement and the performance of RMC+reacq and PMC was only found statistically significant in 1 of 9 comparisons. These results suggest that RMC with selective reacquisition can match the performance of PMC for 3D-MPRAGE and 3D-FLAIR sequences. |
| 16:49 |
1017.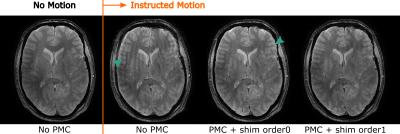 |
Real-time correction of rigid motion and 1st-order shims using
rapid 3D orbital navigators
Malte Riedel1,
Thomas Ulrich1,
and Klaas Pruessmann1
1Institute for Biomedical Engineering, ETH Zurich and University of Zurich, Zurich, Switzerland Keywords: Motion Correction, Brain Rigid head motion, which is a challenging problem in itself, is further accompanied by field variations as the object moves in an inhomogeneous background field, and because pose changes lead to varying susceptibility-induced fields. In contrast to external sensors like optical cameras, navigators are naturally sensitive to field variations. We propose a fast, scan-integrated calibration method to sensitize the 3D orbital navigators to rigid motion and 1st order shim fields. The obtained motion and field parameters are used to correct the scan geometry and shim settings in real-time. The performance is evaluated in phantom and in-vivo studies. |
16:57 |
1018.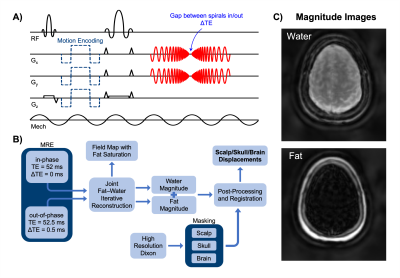 |
Spiral-In/Out MR Elastography with Fat-Water Image
Reconstruction for the Quantification of Skull and Brain Motion
Alexa M Diano1,
Alex M Cerjanic1,2,
Olivia M Bailey1,
Grace McIlvain1,
and Curtis L Johnson1
1Department of Biomedical Engineering, University of Delaware, Newark, DE, United States, 2Department of Medicine, Massachusetts General Hospital, Boston, MA, United States Keywords: Elastography, Traumatic brain injury This study uses a novel multishot spiral-in/out fat-water magnetic resonance elastography (MRE) pulse sequence to simultaneously measure skull and brain motion in vivo in a single acquisition. Results in a healthy volunteer showed reduced transmission of motion from the skull to the brain in all three directions of motion. Additionally, the motion of the skull and brain appear to be in phase, with the scalp moving out of phase and most overall displacement in the direction of actuation. This research provides a basis for future studies quantifying skull-brain coupling with multiple actuation frequencies and excitation directions. |
| 17:05 |
1019.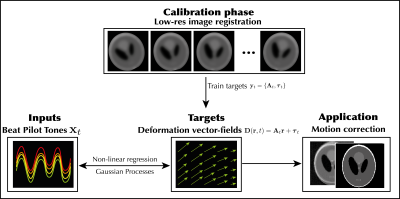 |
Three-dimensional rigid head motion correction using the Beat
Pilot Tone and Gaussian Processes
Niek R.F. Huttinga1,
Suma Anand2,
Cornelis A.T. van den Berg1,
Alessandro Sbrizzi1,
and Michael Lustig2
1Computational Imaging Group for MR therapy & Diagnostics, Department of radiotherapy, University Medical Center Utrecht, Utrecht, Netherlands, 2Electrical Engineering and Computer Sciences, University of California, Berkeley, Berkeley, CA, United States Keywords: Motion Correction, Motion Correction, Brain; Pilot Tone; Surrogate Signals In this work we propose a framework based on Gaussian Processes to extract quantitative motion information from Beat Pilot Tones and subsequently correct rigid head motion in 2D and 3D. In a calibration phase, low-resolution images are acquired and registered to build a training set. Next, Gaussian Processes are trained to infer rigid parameters from multi-channel BPTs, exploiting automatic relevance determination of input channels. In the inference phase, rigid parameters are inferred per readout from the BPT, and high-resolution scans are corrected for motion. In practice the method could reduce the need for anesthetics and/or re-scans in e.g. pediatric patients. |
| 17:13 |
1020.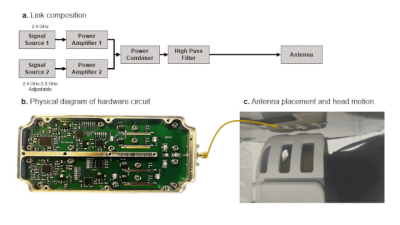 |
Towards contact-free motion sensing technique at low-field MRI
using beat pilot tone
Suen Chen1,
Haoyu Sun1,
Hao Chen1,
Suma Anand2,
Michael Lustig2,
and Zhiyong Zhang1
1School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China, 2Department of Electrical Engineering and Computer Sciences, University of California, Berkeley, Berkeley, CA, United States Keywords: Motion Correction, Low-Field MRI, Pilot Tone To improve the image quality in low field, the conventional method is to repeat scanning many times, and finally increase the SNR by averaging multiple imaging data. Unfortunately, long scan time can make images highly susceptible to motion artifacts. A recent contact-free motion detection technology Beat Pilot Tone (BPT) improves the sensitivity compared with Pilot Tone (PT) and is not limited by Larmor frequency. We introduce BPT in a 0.25T low-field MRI system, and successfully reduce the motion artifacts while improving SNR by binning the continuously acquired data into different motion states in image domain and k-space via BPT signal. |
| 17:21 |
1021.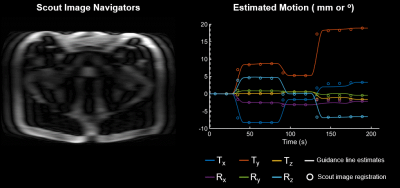 |
Motion Estimation and Retrospective Correction in 2D Cartesian
Turbo Spin Echo Prostate Scans
Bryan Clifford1,
Daniel Polak2,
Wei-Ching Lo1,
Daniel Nicolas Splitthoff2,
Bradley Bolster3,
Vibhas Deshpande4,
Mukesh G. Harisinghani5,
Susie Huang5,
Lawrence L. Wald6,7,
and Stephen Cauley6
1Siemens Medical Solutions, USA, Boston, MA, United States, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Siemens Medical Solutions, USA, Salt Lake City, UT, United States, 4Siemens Medical Solutions, USA, Austin, TX, United States, 5Department of Radiology, Massachusetts General Hospital, Boston, MA, United States, 6Department of Radiology, A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States, 7Harvard-MIT Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States Keywords: Motion Correction, Prostate, MR Value, Motion Correction, Body, Clinical Applications, Prostate Non-rigid patient motion is commonly encountered in clinical settings and can degrade the diagnostic quality of MR exams. We apply the SAMER retrospective method for 2D TSE/FSE prostate imaging to quantify and correct for bulk motion in the abdomen. SAMER utilizes a rapid low-resolution scout scan and a small number of calibration samples for separable motion estimation. In controlled phantom and in vivo experiments, SAMER enabled accurate motion parameter estimation across multiple motion patterns. In addition, we demonstrate improved image homogeneity and spatial resolution when SAMER is used for correction of non-rigid motion in the prostate. |
17:29 |
1022.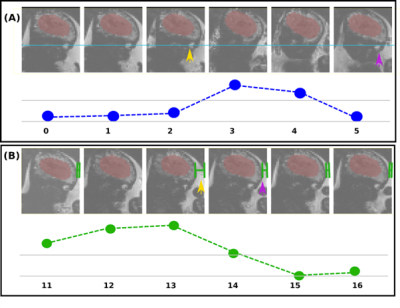 |
Real-time automatic field-of-view adjustment in fetal MR scans
using Gadgetron
Sara Neves Silva1,
Jordina Aviles Verdera1,
Raphaël Tomi-Tricot2,3,
Radhouene Neji2,3,
Thomas Wilkinson1,
Valéry Ozenne4,
Alexander Lewin5,
Lisa Story1,
Enrico De Vita2,
Mary Rutherford1,
Kuberan Pushparajah2,
Jo Hajnal1,
and Jana Hutter1
1Perinatal Imaging & Health, King's College London, London, United Kingdom, 2Biomedical Engineering Department, School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom, 3MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom, 4Université de Bordeaux, Bordeaux, France, 5Forschungszentrum Jülich, Jülich, Germany Keywords: Motion Correction, Fetus MRI provides an ideal tool for characterising fetal brain development and growth. It is, however, a relatively slow imaging technique and therefore extremely susceptible to subject motion. To address this challenge, we are developing an intrinsically motion-robust deep-learning-based fetal MRI method to achieve real-time fetal head tracking and update the acquisition geometry prospectively. Our method uses Gadgetron for real-time reconstruction of the scans and a 3D UNet for fetal head position location and motion estimation. Real-time tracking and correction for functional fetal MRI was demonstrated both in controlled phantom setups and in fetal MRI cases. |
17:37 |
1023.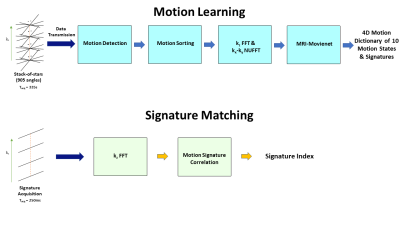 |
MR SIGNATURE MATCHING (MRSIGMA) FOR VOLUMETRIC MRI WITH LESS
THAN 300MS LATENCY ON A 1.5T MR-LINAC SYSTEM
Syed Saad Siddiq1,
Victor Murray1,
Neelam Tyagi1,
Pim T.S. Borman2,
Bas W. Raaymakers2,
Can Wu1,
and Ricardo Otazo1,3
1Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Radiotherapy, University Medical Center Utrecht, Utrecht, Netherlands, 3Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States Keywords: Hybrid & Novel Systems Technology, Radiotherapy The MR-Linac system offers a platform to adapt and monitor radiotherapy of tumors affected by continuous motion in real-time. However, real-time MRI technology is restricted to 2D imaging, which limits performance. MR Signature Matching (MRSIGMA) is a recently developed real-time 3D MRI technique with the MR-Linac as a target. However, MRSIGMA was tested only in a non-real-time scenario. This work develops a real-time implementation of MRSIGMA on the Elekta Unity 1.5T MR-Linac, including raw data transmission to an external computer, deep learning 4D reconstruction and correlation-based matching to demonstrate total imaging latency lower than 300ms. |
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.