Oral Session
Artifacts & Mitigation Strategies
ISMRM & ISMRT Annual Meeting & Exhibition • 03-08 June 2023 • Toronto, ON, Canada

| 08:15 |
1149.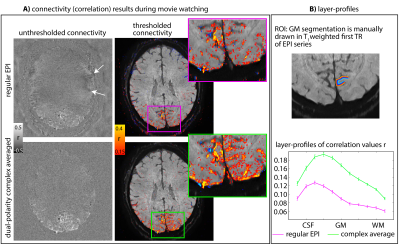 |
Low spatial-frequency ripple artifacts in layer-fMRI EPI:
Identification, cause, and mitigation strategies with
Dual-polarity readout
Renzo Huber1,
Rüdiger Stirnberg2,
David A Feinberg1,3,4,
Samantha J Ma5,
Philipp Ehses2,
Omer Faruk Gulban1,6,
Jonathan R Polimeni7,
Kenshu Koiso1,8,
Emily Ma1,
Alexander JS Beckett3,4,
Tony Stöcker2,
Peter Bandettini9,
and Benedikt A Poser1
1Maastricht University, Maastricht, Netherlands, 2German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 3Helen Wills Neuroscience Institute, University of California, Berkeley, CA, United States, 4Advanced MRI Technologies, Sebastopol, CA, United States, 5Siemens Medical Solutions USA, Inc., Berkeley, CA, United States, 6Brain Innovation, Maastricht, Netherlands, 7Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 8Graduate School of Informatics and Engineering, The University of Electro-Communications, Tokyo, Japan, 9National institutes of HEalth, Bethesda, MD, United States Keywords: Artifacts, fMRI, layer-fMRI, UHF, EPI High-resolution layer-fMRI has great potential to inform network-neuroscience. However, it is limited by EPI artifacts. Here, we discuss a class of fuzzy EPI ghosts arising from asymmetric trapezoidal gradients with ramp sampling. A meta analysis across layer-fMRI datasets finds this artifact everywhere, without exceptions. We believe that this artifact is constraining spatiotemporal resolutions more than SNR. In this abstract we aim to raise awareness for this artifact and evaluate mitigation strategies: dual-polarity EPI. We show that dual-polarity EPI allows layer-fMRI to break the barriers of current resolution limits: It allows 0.53mm imaging at 3T, and whole-brain 0.6mm fMRI at 7T. |
| 08:23 |
1150.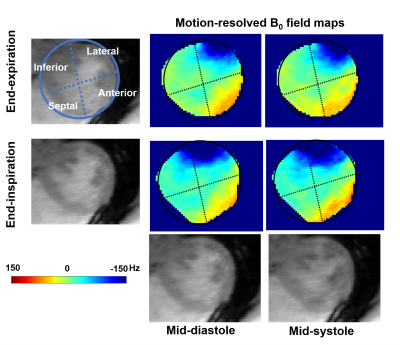 |
The Effect of Respiratory and Cardiac Motion States on B0
Shimming at 3T
Yuheng Huang1,2,
Xingmin Guan1,
Xinheng Zhang1,2,
Liqi(Richard) Tang 1,
Ghazal Yoosefian1,
Xiaoming Bi3,
Fei Han3,
HsuLei Lee4,
Hui Han4,
Anthony Christodoulou4,
Debiao Li4,
Rohan Dharmakumar1,
and Hsin-Jung Yang4 1krannert cardiovascular research center, Indiana University school of medicine, Indianapolis, IN, United States, 2Bioengineering, UCLA, LA, CA, United States, 3Siemens Healthineers, Malvern, PA, United States, 4Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States Keywords: Artifacts, High-Field MRI, Motion-resolved field map, cardiac shimming B0 inhomogeneity imposes imaging artifacts on CMR images and compromises the reliability of popular B0-sensitive sequences such as SSFP at 3T. B0 shimming is the standard way to improve the B0 field. However, motion-induced field inhomogeneity is an unknown factor in routine practice and compromises B0 shimming. Here, we adopted a motion-resolved mGRE CMR sequence to investigate the cardiac B0 field perturbation caused by cardiac and respiratory motion. We found that respiratory motion has more impact on field inhomogeneity. We recommend acquiring a field map for shimming under an end-expiration breath-hold for better shimming and imaging at 3T CMR. |
08:31 |
1151.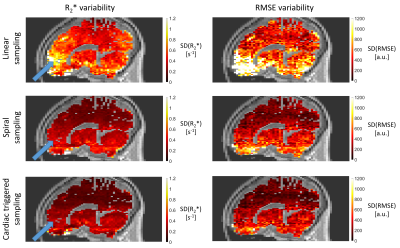 |
Data acquisition strategies to mitigate cardiac-induced noise in
quantitative R2* maps of the brain
Quentin Raynaud1,
Thomas Dardano1,2,
Christopher Roy3,
Jérôme Yerly3,4,
Tobias Kober3,5,6,
Ruud B. van Heeswijk3,
and Antoine Lutti1
1Department for Clinical Neuroscience, Laboratory for Research in Neuroimaging, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 2Physics section, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 3Department of Diagnostic and Interventional Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 4Center for Biomedical Imaging (CIBM), Lausanne, Switzerland, 5Advanced Clinical Imaging Technology, Siemens Healthineers International AG, Lausanne, Switzerland, 6LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland Keywords: Artifacts, Relaxometry Cardiac pulsation enhances the noise level in MR images of the brain and reduces the sensitivity of the data in studies of brain disease. We propose two data acquisition strategies that mitigate cardiac-induced noise in quantitative brain maps of the MRI parameter R2*. The first strategy sets the number of samples at each k-space location according to the local level of cardiac-induced noise. The second strategy adjusts data acquisition in real-time to acquire the data most sensitive to cardiac-induced noise during the diastolic period of the cardiac cycle. |
| 08:39 |
1152.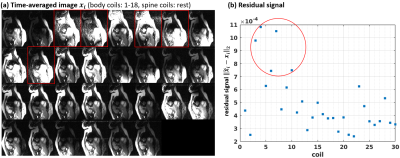 |
Automatic coil selection to suppress motion artifacts in
exercise real-time cine imaging
Chong Chen1,
Yingmin Liu2,
Yu Ding3,
Mathew Tong4,
Yuchi Han4,
and Rizwan Ahmad5
1Biomedical Engineering, The Ohio State Univerity, Columbus, OH, United States, 2Davis Heart and Lung Research Institute, The Ohio Sate University, Columbus, OH, United States, 3Davis Heart and Lung Research Institute, The Ohio State University, Columbus, OH, United States, 4Internal Medicine, The Ohio State University, Colubmus, OH, United States, 5Biomedical Engineering, The Ohio Sate University, Columbus, OH, United States Keywords: Artifacts, Artifacts We propose a novel method to automatically identify and discard coils that strongly contribute to image artifacts. This is achieved by projecting coil images to the space spanned by the ESPIRiT coil sensitivity maps. The proposed method is evaluated using the real-time cine data collected from twelve volunteers during exercise. The artifacts in the reconstructed real-time cine images are suppressed significantly with the proposed coil selection method. |
08:47 |
1153.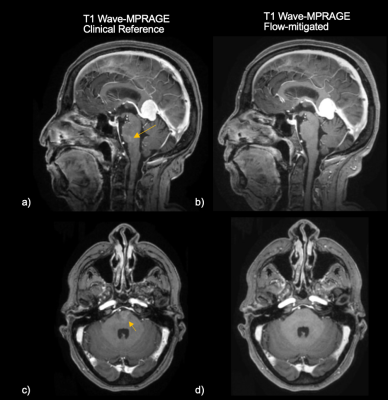 |
Optimized Flow Compensation for Wave-CAIPI Post-Contrast 3D-T1
MPRAGE
Min Lang1,
Azadeh CD Tabari1,
Komal Awan1,
Wei Liu2,
Clifford Bryan3,
Wei-Ching Lo3,
Daniel Nicolas Splitthoff4,
Stephen Cauley1,
Huang Susie1,
and Conklin CD John1
1Massachusetts General Hospital, Boston, MA, United States, 2Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China, 3Siemens Medical Solutions USA, Boston, MA, United States, 4Siemens Healthcare GmbH, Erlangen, Germany Keywords: Artifacts, Data Acquisition, MR Value, Clinical Application, Neuro, Artifacts, Flow, Data Acquisition Flow-related artifacts have been consistently observed in highly accelerated Wave-CAIPI post-contrast 3D-T1 MPRAGE and have an atypical appearance. Such artifacts introduce a diagnostic conundrum as they can mimic enhancing lesions and may require callback for repeat imaging, posing a critical barrier to wider clinical adoption of this technique. To address this, we developed an optimized flow-mitigated Wave-CAIPI post-contrast 3D T1 MPRAGE acquisition, tested it in a novel flow phantom, and deployed it in 17 patients undergoing contrast-enhanced brain MRI. Flow-mitigation was successful at reducing flow-related artifacts in most cases without sacrificing SNR, gray-white matter contrast, or enhancing lesion conspicuity. |
08:55 |
1154.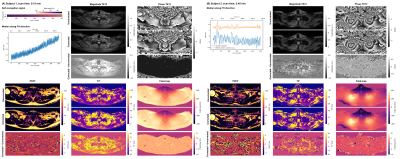 |
Dixon-Based B0-Navigation to Correct B0 Drift and B0
Fluctuations in Radial Stack-Of-Stars Multi-Echo Gradient Echo
Imaging
Jonathan K. Stelter1,
Mingming Wu1,
Johannes Raspe1,
Philipp Braun1,
Christof Boehm1,
Kilian Weiss2,
and Dimitrios C. Karampinos1
1Department of Diagnostic and Interventional Radiology, Technical University of Munich, Munich, Germany, 2Philips Healthcare, Hamburg, Germany Keywords: Artifacts, Motion Correction Multi-echo gradient-echo imaging is known to be affected by temporally varying B0 effects, including B0 drifts and respiratory motion-induced B0 fluctuations. The radial stack-of-stars trajectory enables the oversampling of the k-space center and has been previously employed for B0-navigation without the consideration of fat. The present work develops a methodology for Dixon-based B0-navigation to correct for B0 drift and B0 fluctuations in stack-of-stars multi-echo gradient-echo imaging for body imaging. Simulations and in vivo measurements show the advantage of the Dixon-based B0-navigation in correcting quantification errors in proton density fat fraction, T2* and field-map, when using stack-of-stars multi-echo gradient-echo acquisitions. |
09:03 |
1155.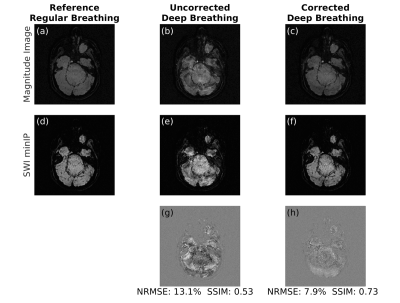 |
Dynamic field correction for improved susceptibility weighted
imaging with FID-navigated 3D EPI
Mustafa Utkur1,2,
Tess E Wallace1,2,
Tobias Kober3,4,5,
Camilo Jaimes1,2,
Simon K Warfield1,2,
Sila Kurugol1,2,
and Onur Afacan1,2
1Radiology, Harvard Medical School, Boston, MA, United States, 2Computational Radiology Laboratory, Boston Children's Hospital, Boston, MA, United States, 3Advanced Clinical Imaging Technology Group, Siemens Healthcare International AG, Lausanne, Switzerland, 4Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 5LTS5, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland Keywords: Artifacts, Susceptibility 3D EPI is a clinically promising alternative for susceptibility weighted imaging due to multi-shot acceleration. However, achieving submillimeter resolution with 3D EPI is challenging as shot-to-shot B0 variations result in artifactual images that limit detection of small veins which can be decisive for detection of the central vein sign in multiple sclerosis. FID navigators have been shown to estimate phase drifts in EPI sequences. We demonstrate that FID-navigated 3D EPI acquisitions enable correction for phase-induced distortions and achieve high-quality submillimeter-resolution susceptibility weighted images. |
| 09:11 |
1156.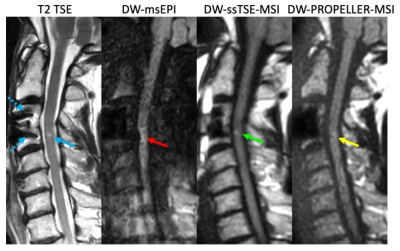 |
Diffusion-weighted MRI of the spinal cord near metal implants: A
rapid TSE approach with multispectral imaging and reduced field
of view
Zhiqiang Li1,
Melvyn B Ooi2,
Rory KJ Murphy1,
John P Karis1,
and Richard D Dortch1
1Barrow Neurological Institute, Phoenix, AZ, United States, 2Philips Healthcare, Houston, TX, United States Keywords: Artifacts, Artifacts, metal, implant, spinal cord, diffusion, multi-spectral imaging Diffusion-weighted (DW) spinal cord MRI based on single-shot EPI suffers from strong geometric distortion and signal loss artifacts. While strategies have been developed to reduce these artifacts in DW-EPI, their application in spinal cord DWI is challenging when metal implants are present near the spine. A multispectral DW-PROPELLER has been proposed to overcome this challenge; however, this requires long scan times. In this work, we developed a single-shot TSE technique with multispectral imaging and reduced FOV to achieve fast speed / increased SNR for spinal cord DWI near metals. Volunteer and patient results demonstrated reduced artifacts and improved speed/SNR performance. |
09:19 |
1157.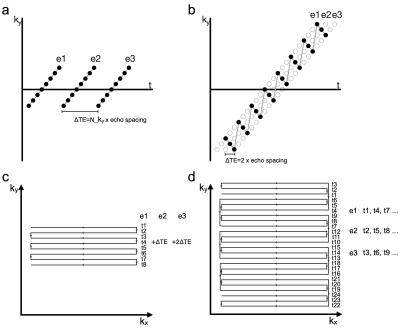 |
Dynamic distortion correction using blip-rewound EPI and joint
multi-echo reconstruction
Wenchuan Wu1
1Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom Keywords: Artifacts, Data Acquisition, EPI distortion In this work, we propose a new method for dynamic distortion correction by integrating a tailored EPI trajectory and a joint multi-echo reconstruction, which permits robust dynamic field mapping and distortion correction without compromising spatial resolution. The performance of the proposed method is demonstrated using in vivo experiments. |
| 09:27 |
1158.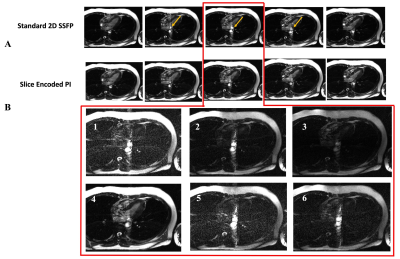 |
Using SMASH Spatial Harmonics to Slice-Encode Outflow Artefacts
in bSSFP Imaging
Fadil Abbas Ali1,
John P Finn1,
and Mark Bydder1 1Radiology, The University of California, Los Angeles, Los Angeles, CA, United States Keywords: Artifacts, Cardiovascular, bSSFP, parallel imaging, spatial harmonics, Cine, Outflow effects In the present of through plane flow in 2D bSSFP imaging, we used the variation coil sensitivity profile along the through-slice direction to estimate the spatial harmonics needed to slice-encode for outflowing spins. Estimating the outer partitions unfolded the outflowing spins from the target center slice with a faster acquisition time than our previous slice-encoding technique. |
| 09:35 |
1159.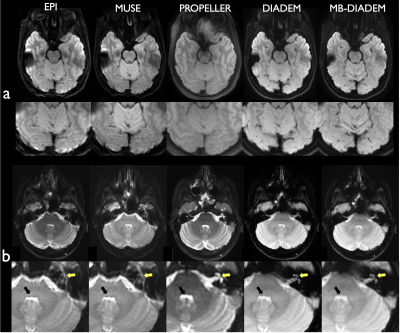 |
Clinical DIADEM diffusion-weighted-imaging (DWI) of the brain:
Comparison with commercially available DWI techniques.
Myung-Ho In1,
Norbert G Campeau1,
Joshua D Trzasko1,
Daehun Kang1,
Kirk M Welker1,
John III Huston1,
Yunhong Shu1,
and Matt A Bernstein1
1Department of Radiology, Mayo Clinic, Rochester, MN, United States Keywords: Artifacts, Diffusion Tensor Imaging A novel distortion-free multi-shot diffusion-weighted-imaging (DWI), termed DIADEM (Distortion-free Imaging: A Double Encoding Method), was compared with commercially available state-of-the-art DWI techniques for clinical brain imaging. High-resolution distortion-free DWI is feasible within clinically acceptable acquisition times using the DIADEM technique, and demonstrated better performance than current commercially available DWI techniques. |
09:43 |
1160.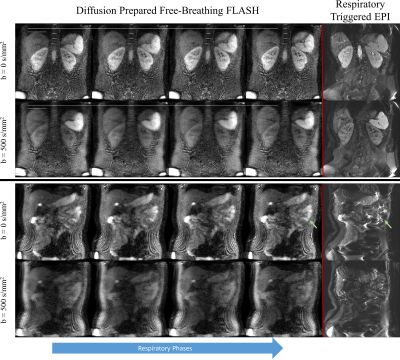 |
Distortionless, free-breathing, and respiratory resolved 3D
diffusion weighted imaging of the abdomen
Philip Kenneth Lee1,
Xuetong Zhou1,2,
Nan Wang1,
and Brian Andrew Hargreaves1,2,3
1Radiology, Stanford University, Stanford, CA, United States, 2Bioengineering, Stanford University, Stanford, CA, United States, 3Electrical Engineering, Stanford University, Stanford, CA, United States Keywords: Pulse Sequence Design, Diffusion/other diffusion imaging techniques, abdomen, free-breathing Abdominal imaging is frequently performed with uncomfortable breath holds, or respiratory triggering to reduce the effects of respiratory motion. Diffusion weighted sequences provide a useful clinical contrast but have prolonged scan times due to low SNR. These scans cannot be reliably completed in a single breath hold, and respiratory triggering has low scan efficiency. We present a respiratory resolved, diffusion-prepared 3D sequence that obtains distortionless diffusion weighted images during free-breathing. We describe techniques to address the myriad of challenges including: 3D shot-to-shot phase correction, respiratory binning, diffusion encoding during free-breathing, and robustness to off-resonance. |
| 09:51 |
1161.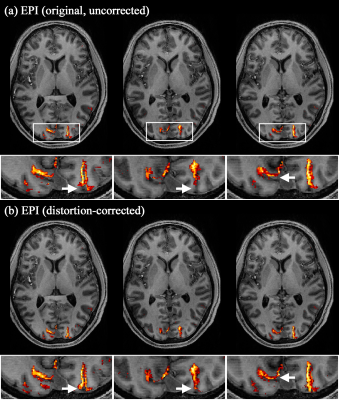 |
A Simultaneous Measurement of Opposite-Phase-Encoding EPI in a
Single fMRI session for the Reduction of Acquisition Time and
SAR at 7T
Seong Dae Yun1,
Erhan Genc2,
and N. Jon Shah1,3,4,5
1Institute of Neuroscience and Medicine 4, INM-4, Forschungszentrum Juelich, Juelich, Germany, 2Department of Psychology and Neurosciences, Leibniz Research Centre for Working Environment and Human Factors (IfADo), Dortmund, Germany, 3Institute of Neuroscience and Medicine 11, INM-11, JARA, Forschungszentrum Juelich, Juelich, Germany, 4JARA - BRAIN - Translational Medicine, Aachen, Germany, 5Department of Neurology, RWTH Aachen University, Aachen, Germany Keywords: Pulse Sequence Design, fMRI, 7T, improved fMRI mapping accuracy, EPI, geometric-distortion correction, opposite phase-encoding and reduced acquisition time In high-resolution fMRI using EPI, geometric distortions typically seen in reconstructed images significantly hinder the accurate mapping of activated voxels. One method to correct for distortions is to acquire EPI data with an opposite phase-encoding direction. However, this method is usually implemented with an additional run of the same protocol, leading to a redundant measurement of parallel imaging calibration scans. Here, we present an EPI scheme that measures the opposite-direction data in a single fMRI session, substantially reducing the total acquisition time. We demonstrate more accurate functional mapping with the distortion correction in submillimetre whole-brain visual fMRI at 7T. |
| 09:59 |
1162.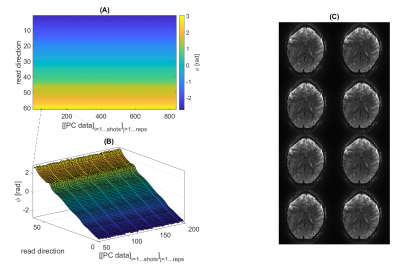 |
Segmented 3D GRE-EPI optimized for BOLD fMRI at 9.4T
Sebastian Mueller1,2,
Jonas Bause1,
Rüdiger Stirnberg3,
and Klaus Scheffler1,2
1High-field Magnetic Resonance Center, Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 2Department for Biomedical Magnetic Resonance, University Hospital Tuebingen, Tuebingen, Germany, 3German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany Keywords: Data Acquisition, fMRI A segmented 3D GRE EPI was adapted and optimized specifically for BOLD fMRI at a 9.4T MR system. While EPI is widely used in fMRI, especially at UHF its application becomes challenging for instance for long echo trains used at high spatial resolution. In this work, investigations on the stability of the system, tSNR, flexible sequence design, different temporal phase correction schemes, and efficient use of the limited SAR budget were performed. Finally, a protocol for BOLD fMRI of the motor cortex at 1.0mm nominal isotropic resolution (FOV=200x215x44mm³) with a volume TR of 3 seconds was set up. |
| 10:07 |
1163.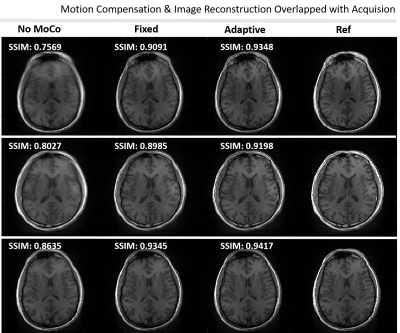 |
Simultaneous Motion Compensation and Image Reconstruction During
Acquisition of 3D Radial MRI
Fatih Calakli1,2,
Tess E. Wallace1,2,
and Simon K. Warfield1,2
1Computational Radiology Laboratory, Boston Children's Hospital, Boston, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States Keywords: Motion Correction, Brain High-resolution, 3D structural scans are susceptible to patient motion as they take several minutes or more to acquire. Radial MRI acquisitions are emerging as a motion-robust alternative to Cartesian trajectories. Most approaches involve co-registration of navigator images once the acquisition is complete. However, the time needed for co-registration of navigator images, coupled with the long reconstruction times required for high-resolution NUFFT, is a challenge for integration of motion-compensated non-Cartesian imaging into clinical protocols. In this work we developed a motion-compensated online gridding algorithm to perform adaptive motion compensated reconstruction overlapped with the acquisition. |
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.