Oral Session
Prostate: Cancer, New Techniques & Beyond
ISMRM & ISMRT Annual Meeting & Exhibition • 03-08 June 2023 • Toronto, ON, Canada

| 16:00 | 0314.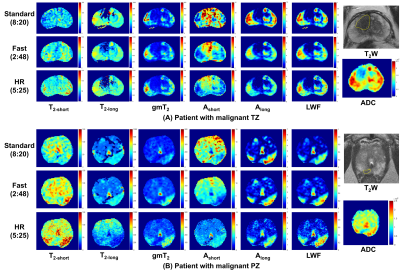 |
Fast and High-resolution Luminal Water Imaging in the Prostate Based on Echo Merging and k-t Undersampling with Reduced Refocusing Flip Angles
Hao Li1,2, Nikita Sushentsev2, Dimitri Kessler2, Shaohang Li3, Kang-Lung Lee2, Andrew Nicholas Priest2,4, Ferdia A Gallagher2, and Tristan Barrett 2
1The Institute of Science and Technology for Brain-inspired Intelligence, Fudan University, Shanghai, China, 2Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 3Shanghai Jiao Tong University University, Shanghai, China, 4Department of Radiology, CUH NHS Foundation Trust, Cambridge, United Kingdom Keywords: Prostate, Cancer The clinical application of luminal water imaging (LWI) has been restricted by its long acquisition time. We present a high acceleration method based on T2 mapping using echo merging plus k-t undersampling with reduced flip angles (TEMPURA), which enables a fast acquisition in 2.8 min as well as a high-resolution acquisition in 5.4 min. Their performance was evaluated on 13 patients with biopsy-proven prostate cancer. Compared with the standard acquisition in 8.3 min, both the fast and high-resolution methods showed a high correlation in LWI measurements and consistent diagnostic performance in detecting malignant lesions. |
| 16:08 | 0315.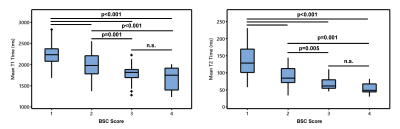 |
Assessment of Background Heterogeneity of Prostate Peripheral Zone for Cancer Detectability using Magnetic Resonance Fingerprinting
Peter Qiao1, Donald Brennan1, Sree Harsha Tirumani2,3, Yong Chen2,3, Mark Griswold2,3, and Leonardo Kayat Bittencourt2,3
1School of Medicine, Case Western Reserve University, Cleveland, OH, United States, 2Department of Radiology, Case Western Reserve University, Cleveland, OH, United States, 3Department of Radiology, University Hospitals Cleveland Medical Center, Cleveland, OH, United States Keywords: Prostate, Cancer In this pilot study, we assessed the background signal change (BSC) of the prostate peripheral zone based on quantitative T1 and T2 measurement obtained using Magnetic Resonance Fingerprinting (MRF) and correlated with a previously-developed qualitative BSC score. Our results demonstrate the importance of prostate BSC toward reducing uncertainty in prostate MRI. Compared to the qualitative BSC score, the MRF-derived measurements provide a more reproducible and objective assessment of background heterogeneity of the prostate. BSC scoring has great potential in risk-stratifying patients with incidentally-detected clinically significant prostate cancer and yielding better biopsy decision-making for patients with negative MRI. |
| 16:16 | 0316.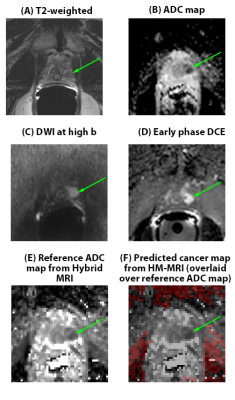 |
THE ADDED VALUE OF HYBRID MULTIDIMENSIONAL MRI TO MULTIPARAMETRIC MRI IN DIAGNOSING CLINICALLY SIGNIFICANT PROSTATE CANCERS
Grace Lee1, Aritrick Chatterjee1, Ibrahim Karademir1, Roger Engelmann1, Ambereen Yousuf1, Mihai Giurcanu2, Carla Harmath1, Gregory Karczmar1, and Aytekin Oto1 1RADIOLOGY, University of Chicago, Chicago, IL, United States, 2BIOSTATISTICS, University of Chicago, Chicago, IL, United States Keywords: Prostate, Quantitative Imaging, Multiparametric MRI 1. In a retrospective review of 61 men by four readers, two inexperienced-readers had higher accuracy using multiparametric MRI+Hybrid-Multidimensional MRI (mpMRI+HM-MRI)=82%,81% versus Multiparametric MRI=77%, 71% (p=.006,<.001) and higher specificity=89%,88% versus 84%,75% (p=.009, p<.001) on a per-sextant analysis. 2. Multiparametric MRI+Hybrid-Multidimensional MRI increased trainee specificity=46% versus Multiparametric MRI=7% (p<.001) on a per-patient analysis. 3. Multiparametric MRI+Hybrid-Multidimensional MRI increased interreader Kappa agreement=0.36 versus Multiparametric MRI (mpMRI) Kappa=0.17 (p=0.009) in diagnosing clinically significant prostate cancer (CS PCa). |
| 16:24 | 0317.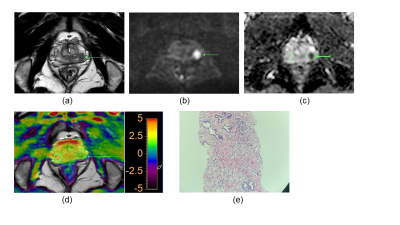 |
Amide Proton Transfer Weighted Imaging to differentiate prostate suspicious nodules: Comparison with PI-RADS v2.1 score
Guanyong He1, Liying Han1, Ronghua Yan1, Hao Li1, Zhigang Wu2, Qingping Gu2, and Guanxun Cheng1
1Peking University Shenzhen Hospital, Shenzhen, China, 2Clinical & Technical Support, Philips Healthcare (Shenzhen) Ltd, Shenzhen, China Keywords: Prostate, CEST & MT, APT Diagnosis of prostate cancer (PCa) remains a challenge since PCa often occurs simultaneously with benign prostatic hyperplasia and has similar nodule appearance on T2-weighted imaging and diffusion weighted imaging. Amide proton transfer weighted (APTw) imaging is an endogenous biomarker to detect proteins and peptides in tissues non-invasively. Results of this study indicate the APTw ratio (rAPTw) values of PCa were significantly higher than those of prostate benign nodules. Therefore, rAPTw value is potentially a promising and valuable non-invasive biomarker in differentiating PCa from benign nodules. |
| 16:32 | 0318.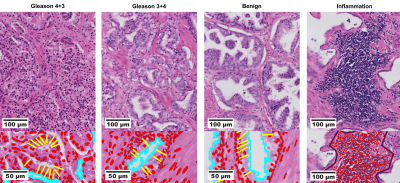 |
Decomposition of clinical ADC into intracellular and extracellular-extravascular contributions in prostate cancer using histology
Alonso Garcia-Ruiz1, Francesco Grussu1, Snigdha Sen2, Chen Jin2, Alex Freeman3, Aiman Haider3, Shonit Punwani4, Daniel C. Alexander2, Raquel Perez-Lopez1, and Eleftheria Panagiotaki2
1Radiomics Group, Vall d'Hebron Institute of Oncology, Barcelona, Spain, 2Department of Computer Science, Centre for Medical Image Computing, University College London, London, United Kingdom, 3Department of Pathology, University College London Hospitals NHS Foundation Trust, London, United Kingdom, 4Centre for Medical Imaging, University College London, London, United Kingdom Keywords: Prostate, Cancer, Histology Diffusion MRI has shown promising results for characterizing prostate cancer. However, diagnostic clinical apparent diffusion coefficient (ADC) has limited specificity and interpretability. Towards addressing these limitations, we aim to unravel clinical ADC into microstructural components using histology. Histology from two prostatectomies with prostate cancer (Gleason 3+4, 4+3) were analysed in benign, inflammation and cancer regions. Cell and tissue properties were used to decouple clinical ADC into intracellular, extracellular-extravascular ADCs. Results revealed significantly low intracellular ADC in cancer. Low ADC was also found for inflammation, which could explain ADC’s low specificity, demonstrating the added value of histology data for clinical ADC. |
| 16:40 | 0319.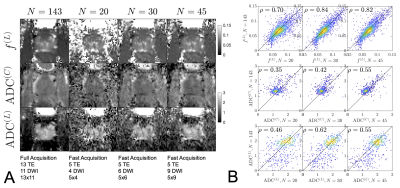 |
Direct measurement of luminal diffusivity in prostate: Prostate diffusion at an echo time of 400 ms.
Gregory Lemberskiy1,2, Santiago Coelho1, Mary Bruno1, Els Fieremans1, and Dmitry S Novikov1 1New York University Grossman School of Medicine, New York, NY, United States, 2Microstructure Imaging, INC, New York, NY, United States Keywords: Prostate, Prostate, Diffusion We showcase, for the first time, a direct/model-free measurement of luminal diffusivity in prostate, where ADC contrast no longer contains the cellular contribution, and only describes features of the glandular lumen, which prominently shrink with increasing Gleason score. We show that with a 4m30s protocol, the luminal volume fraction, cellular and luminal diffusivities can be obtained with high accuracy/precision at 1.5x1.5x3.0 mm3, suggesting that this approach can feasibly replace the current state-of-the-art, ~6 minute, prostate diffusion acquisition. |
| 16:48 | 0320.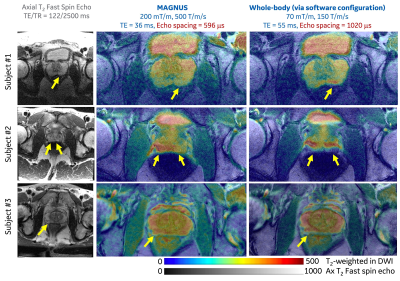 |
Human Prostate MRI at ultra-high-performance gradient of 200 mT/m and 500 T/m/s
Ante Zhu1, Matthew Tarasek1, Yihe Hua1, Eric Fiveland1, Desmond T.B. Yeo1, Stephan E. Maier2, Yousef Mazaheri3, Filip Szczepankiewicz4, Carl-Fredrik Westin2, Clare Tempany2, Oguz Akin3, and Thomas K.F. Foo1
1GE Research, Niskayuna, NY, United States, 2Brigham and Women's Hospital, Boston, MA, United States, 3Memorial Sloan Kettering Cancer Center, New York, NY, United States, 4Lund University, Lund, Sweden Keywords: Prostate, Diffusion/other diffusion imaging techniques, Tumor, Microstructure Diffusion MRI has been shown to yield imaging biomarkers for prostate cancer identification and grading. However, the image quality remains poor due to the limited gradient performance of clinical whole-body MRI systems. We assessed the technical feasibility of human prostate MRI in a 42-cm inner-diameter gradient coil operating at maximum gradient amplitude of 200 mT/m and slew rate of 500 T/m/s. No sensation of peripheral nerve stimulation was reported. Significantly reduced image distortion and higher image signal-to-noise ratios were achieved in diffusion MRI due to shorter echo times and shorter echo spacings, compared to diffusion MRI at whole-body MRI systems. |
| 16:56 | 0321.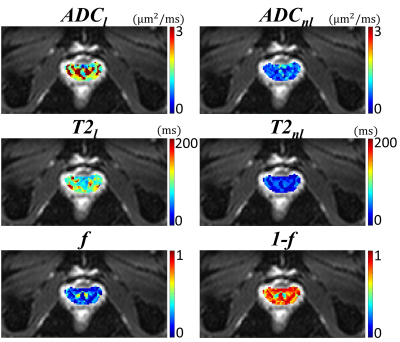 |
Multi-echo Diffusion-weighted Imaging for Quantifying Luminal Water Fraction in the Prostate
Kaibao Sun1, Guangyu Dan1,2, Muge Karaman1,2, Qingfei Luo1, and Xiaohong Joe Zhou1,2,3
1Center for MR Research, University of Illinois at Chicago, Chicago, IL, United States, 2Department of Biomedical Engineering, University of Illinois at Chicago, Chicago, IL, United States, 3Departments of Radiology and Neurosurgery, University of Illinois at Chicago, Chicago, IL, United States Keywords: Prostate, Quantitative Imaging Prostate cancer remains one of the leading causes of cancer-related deaths in men. Reduced luminal water fraction has been observed in prostate cancer using diffusion MRI, but the image acquisition takes a long time. We herein introduce a multi-echo DWI sequence that is capable of quantifying luminal water fraction with a substantially improved time efficiency. The sequence incorporated multiple readout echo-trains into diffusion-weighted EPI, together with a 2D RF excitation pulse to reduce the FOV, thereby enabling multiple TEs in one shot. With this sequence, we have evaluated the volume fractions of lumen and non-lumen tissues in the human prostate. |
| 17:04 | 0322.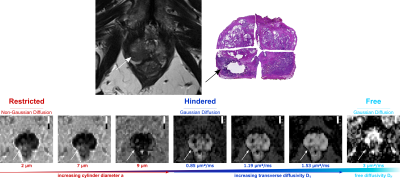 |
Investigating tissue microstructure of prostate cancer using Linear Multi-scale Modeling of diffusion MRI data
Barbara Daria Wichtmann1, Niklas Westhoff2, Cleo-Aron Weis3, Ralph Strecker4, Thorsten Feiweier5, Steffen Albert6,7, Moritz Wolter8, Frank Zöllner6,7, Bettina Baeßler9, Aapo Nummenmaa10, Qiuyun Fan10,11, Susie Huang10,12, and Ulrike Attenberger1
1Diagnostic and Interventional Radiology, University Hospital Bonn, Bonn, Germany, 2Department of Urology and Urosurgery, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany, 3Institute of Pathology, University Hospital Heidelberg, Heidelberg, Germany, Heidelberg, Germany, 4EMEA Scientific Partnerships, Siemens Healthcare GmbH, Erlangen, Germany, 5MR Application Development, Siemens Healthcare GmbH, Erlangen, Germany, 6Computer Assisted Clinical Medicine, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany, 7Mannheim Institute for Intelligent Systems in Medicine, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany, 8High Performance Computing & Analytics Lab, University Bonn, Bonn, Germany, 9Institute of Diagnostic and Interventional Radiology, University Hospital Würzburg, Würzburg, Germany, 10A. A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 11Department of Biomedical Engineering, College of Precision Instruments and Optoelectronics Engineering, Tianjin University, Tianjin, China, 12Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States Keywords: Prostate, Cancer Linear Multi-scale Modeling (LMM) is an advanced diffusion-weighted imaging(DWI) technique that uses multi-shell, multi-diffusion-time DWI data to estimate tissue microstructure parameters, including volume fractions of restricted and hindered water compartments over a range of length scales and orientation distribution information. Here,we apply the LMM framework to characterize prostate cancer(PCa) lesions and correlate our results with histology. Within the histopathologically proven cancerous lesions we observed a significantly increased fraction of restricted diffusion, particularly within the 2μm and 7μm sized water compartments. LMM may enable the development of distinct diffusion microstructural signatures of PCa to facilitate diagnosis of clinically significant PCa lesions. |
| 17:12 | 0323.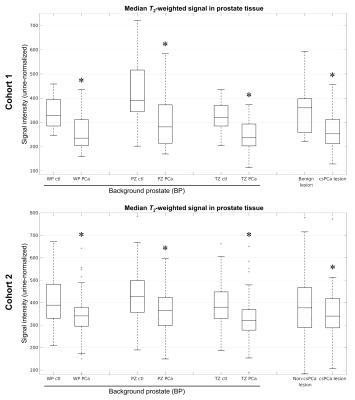 |
Background prostate tissue is quantitatively abnormal on T2-weighted MRI in patients with clinically significant prostate cancer
Christopher C Conlin1, Roshan Karunamuni2, Troy S Hussain2, Allison Y Zhong3, Karoline Kallis2, Deondre D Do2,4, Asona J Lui2, Garnier Mani3, Courtney Ollison5, Mariluz Rojo Domingo4, Ahmed Shabaik6, Christopher J Kane7, Aditya Bagrodia7, Rana R McKay7,8, Joshua M Kuperman1, Rebecca Rakow-Penner1, Michael E Hahn1, Anders M Dale1,9,10, and Tyler M Seibert1,2,4
1Department of Radiology, University of California San Diego, La Jolla, CA, United States, 2Department of Radiation Medicine and Applied Sciences, University of California San Diego, La Jolla, CA, United States, 3School of Medicine, University of California San Diego, La Jolla, CA, United States, 4Department of Bioengineering, University of California San Diego, La Jolla, CA, United States, 5Department of Biology, San Diego State University, San Diego, CA, United States, 6Department of Pathology, University of California San Diego, La Jolla, CA, United States, 7Department of Urology, University of California San Diego, La Jolla, CA, United States, 8Department of Medicine, University of California San Diego, La Jolla, CA, United States, 9Department of Neurosciences, University of California San Diego, La Jolla, CA, United States, 10Halıcıoğlu Data Science Institute, University of California San Diego, La Jolla, CA, United States Keywords: Prostate, Cancer, T2-weighted MRI; Background prostate; Benign-appearing prostate In this study, we examined whether patients with clinically significant prostate cancer (csPCa) have abnormal T2-weighted signal in prostate tissue outside of index lesions identified on MRI—i.e., in the background prostate (BP). In two independent patient cohorts, normalized T2-weighted signal was systematically lower in the BP of subjects with csPCa compared to those without. Reduced T2-weighted BP signal indicated the presence of csPCa with accuracy comparable to lesion-based measurements. Consideration of T2-weighted signal in the whole prostate improved patient-level detection of csPCa over DWI alone, suggesting that it provides complementary diagnostic value. |
| 17:20 | 0324.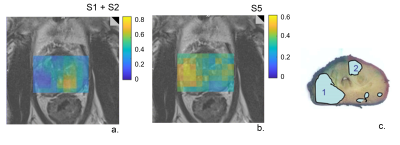 |
Prostate cancer characterization with a data-driven analysis of multi-center 3D 1H-MRSI data using Multivariate Curve Resolution
Angeliki Stamatelatou1, Carlo Giuseppe Bertinetto2, Jeroen J Jansen2, Geert J Postma2, Kirsten Margrete Selnæs3, Tone Frost Bathen3, Arend Heerschap1, and Tom WJ Scheenen1
1Department of Medical Imaging, Radboud University Medical Center, Nijmegen, Netherlands, 2Department of Analytical Chemistry & Chemometrics, Radboud University, Nijmegen, Netherlands, 3Department of Circulation and Medical Imaging, Norwegian University of Technology and Science, Trondheim, Norway Keywords: Prostate, Spectroscopy, multi-center, cancer, aggressiveness Location and aggressiveness of cancer lesions in the prostate were assessed with a Multivariate Curve Resolution (MCR) approach. This data-driven method was trained on 3D 1H MRSI data from 63 patients from 6 institutions to extract common spectroscopic components without needing prior knowledge. MCR identified 5 components related to known prostate metabolites, residual lipid and water signals. The relative amounts of these components robustly separated cancer from benign tissue in a test set of 43 other subjects from 8 centers, and showed a strong correlation with cancer aggressiveness. This approach facilitates automatic analysis of prostate MRSI, avoiding subjective human intervention. |
17:28 |
0325.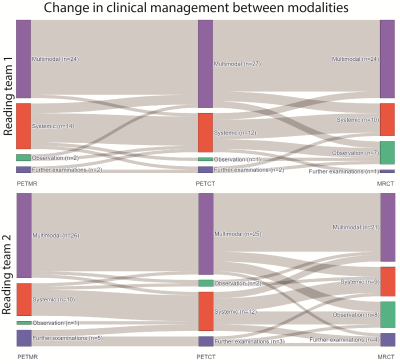 |
18F-PSMA-1007 PET/MR compared to PET/CT in prostate cancer recurrence: impact on clinical management
Bendik Skarre Abrahamsen1, Trond Velde Bogsrud2,3, Eivor Hernes4, Torgrim Tandstad5,6, Sverre Langørgen7, Miguel Castillejo2, Kirsten Margrete Selnæs7, Ingerid Skjei Knudtsen1, Thomas Morten Ingmar Keil7, Håkon Johansen7, Tone Frost Bathen1,7, and Mattijs Elschot1,7
1Derpartment of Circulation and Medical Imaging, Norwegian University of Science and Technology, Trondheim, Norway, 2PET Imaging Centre, University Hospital of North Norway, Tromsø, Norway, 3PET-Centre, Aarhus University Hospital, Aarhus, Denmark, 4Department of nuclear medicine, Oslo University Hospital, Oslo, Norway, 5The Cancer Clinic, St. Olavs Hospital, Trondheim, Norway, 6Department of Clinical and Molecular Medicine, Norwegian University of Science and Technology, Trondheim, Norway, 7Department of Radiology and Nuclear Medicine, St. Olavs Hospital, Trondheim, Norway Keywords: Prostate, PET/MR For patients with suspected prostate cancer recurrence, we examined the added value of 18F-PSMA-1007 PET to MR and CT imaging, and whether choice of PET modality, i.e. PET/MR or PET/CT, had an impact on clinical management. An intention-to-treat analysis was performed using findings from MR and CT alone, MR and CT with PET from PET/CT, and MR and CT with PET from PET/MR. Addition of 18F-PSMA PET changed clinical management in over 40% of patients. Differences in clinical management between PET/CT and PET/MR were smaller than adding any PET modality to MR and CT. |
| 17:36 | 0326.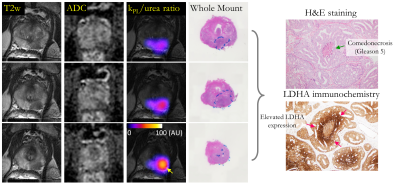 |
Hyperpolarized 13C Pyruvate + Urea Dual Agent MRI Detects Hypoxia-Driven Metabolism Perfusion Mismatch in Patient w/ Aggressive Prostate Cancer
Hsin-Yu Chen1, Ivan de Kouchkovsky2, Hao G. Nguyen2, Bradley A. Stohr2, Daniel Gebrezgiabhier1, Romelyn Delos Santos1, Lucas Carvajal1, Michael A. Ohliger1, Zhen J. Wang1, Hecong Qin1, Xiaoxi Liu1, Jeffry P. Simko2, Jeremy W. Gordon1, Peder E.Z. Larson1, Robert A. Bok1, Rahul Aggarwal2, John Kurhanewicz1, and Daniel B. Vigneron1 1Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, San Francisco, CA, United States Keywords: Prostate, Cancer The first-in-human hyperpolarized [1-13C]pyruvate+[13C,15N2]urea dual-agent MRI demonstrated the safety and feasibility of simultaneous characterization of prostate cancer metabolism and blood flow as a two-minute addition to the standard 1H-multiparametric MRI. Metabolism-perfusion mismatch (i.e. elevated pyruvate-lactate conversion and decreased urea perfusion) in subregions of the high-grade prostate tumor was in agreement with the histopathological and immunochemical markers that reflected lethal phenotypes and their associated hypoxic tumor microenvironment. Correlative analysis of urea and Gadolinium-derived pharmacokinetic parameters found low correlation between the two, which indicates that HP 13C urea’s unique contrast mechanism offers independent information inaccessible through conventional 1H DCE-MRI. |
| 17:44 | 0327.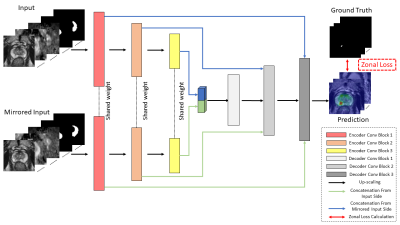 |
Anatomical-aware Siamese Deep Network for Prostate Cancer Detection on Multi-parametric MRI
Haoxin Zheng1,2, Miao Qi2,3, Alex Ling Yu Hung1,2, Kai Zhao2, Steven Raman2, and Kyunghyun Sung2
1Computer Science, University of California, Los Angeles, Los Angeles, CA, United States, 2Radiological Science, University of California, Los Angeles, Los Angeles, CA, United States, 3Radiology, The First Affiliated Hospital of China Medical University, Shenyang, China Keywords: Prostate, Cancer, Machine Learning The study aimed to build a deep-learning-based prostate cancer (PCa) detection model integrating the anatomical priors related to PCa’s zonal appearance difference and asymmetric patterns of PCa. A total of 220 patients with 246 whole-mount histopathology (WMHP) confirmed clinically significant prostate cancer (csPCa), and 432 patients with no indication of lesions on multi-parametric MRI (mpMRI) were included in the study. A proposed 3D Siamese nnUNet with self-designed Zonal Loss was implemented, and results were evaluated using 5-fold cross-validation. The proposed model that is aware of PCa-related anatomical information performed the best on both lesion-level detection and patient-level classification experiments. |
| 17:52 | 0328.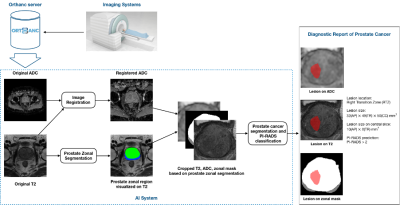 |
An AI-based Pipeline for Prostate Cancer PI-RADS Reporting on Multiparametric MRI using MiniSegCaps Network
Wenting Jiang1, Yingying Lin1, Varut Vardhanabhuti1, and Peng Cao1
1Department of Diagnostic Radiology, the University of Hong Kong, Hong Kong, Hong Kong Keywords: Multimodal, Cancer Prostate Imaging Reporting and Data System (PI-RADS) on multiparametric MRI (mpMRI) provides fundamental MRI interpretation guidelines but suffers from inter-reader variability. Deep learning networks show great promise in automatic lesion segmentation and classification, which help to ease the burden on radiologists and reduce inter-reader variability. In this study, we proposed a novel multi-branch network, MiniSegCaps, for prostate cancer segmentation and PI-RADS classification on mpMRI, and a graphical user interface (GUI) integrated into the clinical workflow for diagnosis reports generation. Our model achieved the best performance in prostate cancer segmentation and PIRADS classification compared with state-of-the-art methods. |
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.