Oral Session
Advanced Physiological Characterization of the Heart
ISMRM & ISMRT Annual Meeting & Exhibition • 03-08 June 2023 • Toronto, ON, Canada

| 13:45 |
0169.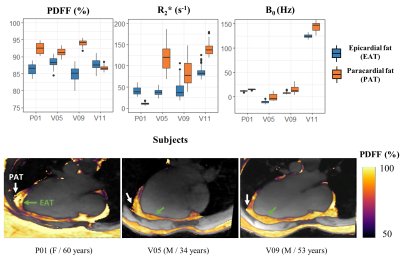 |
Cardiac adipose tissue proton-density fat fraction
quantification by free-running cardiac Dixon at 3T
Pierre Daudé1,2,
Thomas Troalen3,
Adèle L C Mackowiak4,5,6,
Emilien Royer1,2,
Davide Piccini4,7,
Jérôme Yerly4,8,
Josef Pfeuffer9,
Frank Kober1,2,
Sylviane Confort Gouny1,2,
Monique Bernard1,2,
Matthias Stuber4,8,
Jessica A M Bastiaansen5,6,
and Stanislas Rapacchi1,2
1Aix-Marseille Univ, CNRS, CRMBM, Marseille, France, 2APHM, Hôpital Universitaire Timone, CEMEREM, Marseille, France, 3Siemens Healthcare SAS, Saint-Denis, France, 4Department of Diagnostic and Interventional Radiology, Lausanne University Hospital, Lausanne, Switzerland, 5Department of Diagnostic, Interventional and Pediatric Radiology (DIPR), Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland, 6Translation Imaging Center (TIC), Swiss Institute for Tranlational and Entrepreneurial Medicine, Bern, Switzerland, 7Advanced Clinical Imaging Technology, Siemens Healthineers International AG, Lausanne, Switzerland, 8Center for Biomedical Imaging, Lausanne, Switzerland, 9Siemens Healthcare, MR Application Development, Erlangen, Germany Keywords: Fat, Quantitative Imaging Free-running cardiac Dixon-MRI has the potential for motion-resolved R2* and PDFF quantification to explore cardiac fat accumulation and alteration in metabolic diseases. This study combines a self-navigated 3D radial sequence with 13 echoes using fast bipolar signal-readouts, a k-space trajectory correction, a compressed sensing reconstruction, and IDEAL fat-water separation to obtain precise ( limits of agreement: ±1.2 % and 5.0 s-1) and highly spatially and motion-resolved quantitative PDFF and R2* maps of the whole heart. We report here the first in vivo characterization of metabolically-active epicardial fat (PDFF: 81.6±9.6 %), distinct from adjacent paracardial fat. |
| 13:53 |
0170. |
Evaluation of Cardiac Microstructure in Patients with Severe
Aortic Stenosis Using Diffusion Tensor MRI with Submillimeter
Resolution
Christopher A. Rock1,2,
Iris Y. Chen2,
Anne L. Philip1,
Boris Keil2,3,
Christopher T. Nguyen2,4,5,
and David E. Sosnovik 1,2,5
1Cardiovascular Research Center, Massachusetts General Hospital, Boston, MA, United States, 2A.A Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 3Institute of Medical Physics and Radiation Protection, TH Mittelhessen University of Applied Sciences, Giessen, Germany, 4Cardiovascular Innovation Research Center, Heart, Vascular, and Thoracic Institute, Cleveland Clinic, Cleveland, OH, United States, 5Health Sciences and Technology Program, Harvard - Massachusetts Institute of Technology, Cambridge, MA, United States Keywords: Myocardium, Diffusion Tensor Imaging This study describes a method to acquire submillimeter resolution diffusion tensor MRI scans of the human heart in vivo. Imaging was performed in healthy controls and patients with severe high flow-high gradient aortic stenosis. The images were processed to generate helix angle maps of cardiomyocyte orientation and compared. The range of helix angles across the myocardium was higher in subjects with aortic stenosis and the relative helix angle slope was also increased in patients with aortic stenosis. |
14:01 |
0171.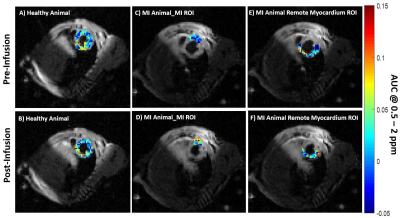 |
In-Vivo CEST MRI to assess and identify myocardial infarction by
using natural D- glucose as a contrast agent
Ajay Peddi1,
Daniel Schache1,
Ali Nahardani2,
Michael Kuhlmann3,
Moritz Wildgruber1,4,
Cornelius Faber1,
and Verena Hoerr1,2
1Translational Research Imaging Center, Clinic of Radiology, University of Münster, Münster, Germany, 2Heart Center Bonn, Department of Internal Medicine II, University Hospital Bonn, Bonn, Germany, 3European Institute for Molecular Imaging, University of Münster, Münster, Germany, 4Clinic and Polyclinic for Radiology, University Hospital of München, München, Germany Keywords: Myocardium, CEST & MT The current preclinical study aimed to explore the application of natural D-glucose as an infusible biodegradable MRI contrast agent for imaging of myocardial infarction (MI) by glucose weighted CEST MRI (glucoCEST). To this end, in a mouse model of MI, the infarct region was first identified and verified by late gadolinium enhancement MRI and histology, respectively. In-Vivo glucoCEST MTRasym maps showed substantial differences before and after glucose infusion according to the myocardial viability. Statistical analysis verified that glucoCEST contrast could distinguish significantly between MI region, remote myocardium as well as healthy myocardium. |
| 14:09 |
0172.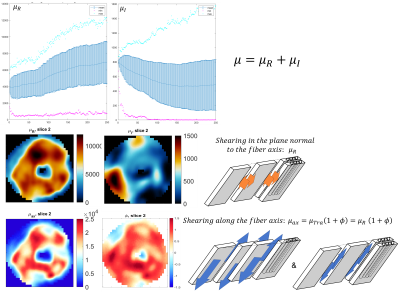 |
Anisotropic stiffness of ex vivo swine heart estimated by
transversely isotropic nonlinear inversion MRE at 2mm isotropic
voxel resolution
Cyril Tous1,2,
Guillaume Flé3,
Matthew McGarry4,
Philip Bayly5,
Keith Paulsen4,6,
Curtis Johnson7,
Matthias Stuber1,2,
and Elijah Van Houten8
1Diagnostic and Interventional Radiology, Lausanne University Hospital (CHUV), Lausanne, Switzerland, 2Center for Biomedical Imaging (CIBM), Lausanne, Switzerland, 3Université de Montréal, Montréal, QC, Canada, 4Thayer school of engineering, Dartmouth College, Hanover, NH, United States, 5McKelvey School of Engineering, Washington University, Saint Louis, MO, United States, 6Dartmouth-Hitchcock Medical Center, Lebanon, NH, United States, 7Biomedical Engineering, University of Delaware, Newark, DE, United States, 8Mechanical Engineering, Université de Sherbrooke, Sherbrooke, QC, Canada Keywords: Heart, Elastography MR elastography (MRE) must account for the anisotropic nature of myocardial tissue to accurately quantify stiffness. The constitutive matrix for this material was rotated to align with the fibers. One ex vivo swine heart was scanned with DTI and MRE sequences at 2 isotropic voxel resolution. Transversely isotropic viscoelastic stiffness was reconstructed using the Non-Linear Inversion (NLI) algorithm. Elastic properties (shear and Young’s modulus, tensile and shear anisotropy) were segment dependent, in agreement with the myocyte sheetlet formation, which varies in size and spacing according to the myocardial segment. Similarities between MRE and DTI metrics could be observed. |
14:17 |
0173.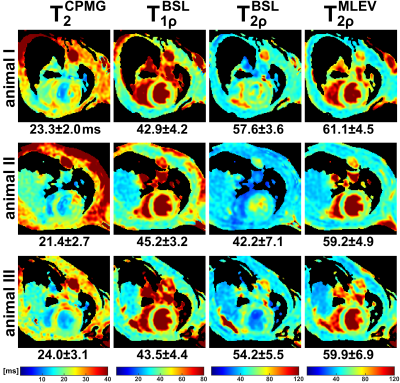 |
Myocardial T2ρ Mapping in Small Animals: Comparison of Balanced
Spin-Lock and Malcolm-Levitt Preparations
Maximilian Gram1,2,
Petra Albertova1,2,
Fabian Tobias Gutjahr2,
Peter Michael Jakob2,
Wolfgang Rudolf Bauer3,4,
Peter Nordbeck1,4,
and Martin Christa1,4
1Department of Internal Medicine I, University Hospital Würzburg, Würzburg, Germany, 2Experimental Physics 5, University of Würzburg, Würzburg, Germany, 3Department of Internal Medicine I, Divisions of Cardiology and Nephrology, University Hospital Würzburg, Würzburg, Germany, 4Comprehensive Heart Failure Center (CHFC), Würzburg, Germany Keywords: Relaxometry, Quantitative Imaging, Spin-Lock, T1ρ, T2ρ In this work, we propose myocardial T2ρ mapping as a potential and more robust alternative to conventional T2 quantification. Our new approach for T2ρ imaging, which is based on Malcolm-Levitt preparations with zero inter-pulse delays, was compared with established pulse sequences for T2, T1ρ and T2ρ in both phantom and in vivo experiments. In summary, the new preparatory pulse sequence was shown to meet the demanding requirements of myocardial T2ρ mapping at high magnetic field strength and to outperform conventional T2ρ preparations using balanced spin-locking. |
| 14:25 |
0174.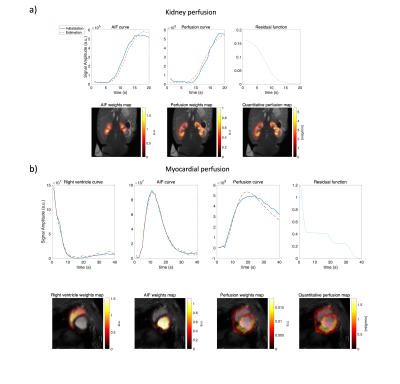 |
A constrained decomposition method for myocardial perfusion
quantification using hyperpolarized MRI
Yupeng Zhao1,
Rie Beck Olin1,
Esben Søvsø Szocska Hansen2,
Christoffer Laustsen2,
Jan Henrik Ardenkjær-Larsen1,
and Lars G. Hanson1,3
1Technical University of denmark, Kongens Lyngby, Denmark, 2MR Research Centre, Aarhus University, Aarhus, Denmark, 3Danish Research Centre for Magnetic Resonance, Hvidovre, Denmark Keywords: Myocardium, Perfusion As an alternative to Gadolinium, metabolically inert hyperpolarized contrast agents have been used in perfusion studies. For myocardial perfusion measurements, it is challenging to reliably obtain perfusion-related signals due to partial volume effects. We propose a constrained decomposition approach, enforcing prior knowledge of the residue function being temporally decreasing. The method is capable of separating the arterial and perfusion components and the quantified perfusion map is not visibly affected by partial volume effects. |
| 14:33 |
0175.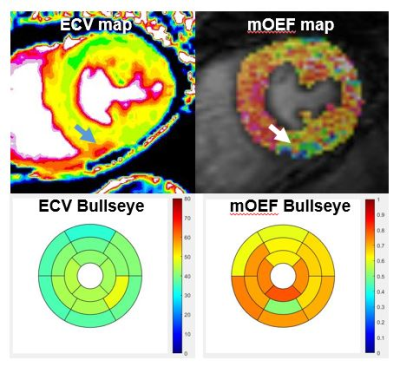 |
Myocardial oxygen extraction fraction moderately correlates with
fibrosis burden in patients after heart transplant: a MR
metabolism study
Jing Wang1,
Ran Li2,
Xiaona Fu3,
Yang Yang4,
Xiaojie Sun3,
Shenglei Shu3,
Jie Zhao3,
Xiangchuang Kong3,
and Jie Zheng2 1Radiology, Union hospital, Wuhan, China, 2Mallinckrodt Institute of Radiology, Washington University in St Louis, St. Louis, MO, United States, 3Union hospital, Wuhan, China, 4Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States Keywords: Myocardium, Transplantation, oxygen metabolism Microvascular dysfunction and excessive fibrous burden are independent prognostic factors after heart transplantation. A new novel cardiovascular MR technique for the measurement of myocardial oxygen extraction fraction was evaluated for a feasibility study in 10 patients after heart transplantation. It was observed that oxygen extraction fraction was significantly correlated with myocardial extracellular volume (diffuse fibrosis indicator). Further research with more patients is warranted to explore this new CMR metabolism index for early diagnosis of cardiac dysfunction in these patient cohorts. |
| 14:41 |
0176.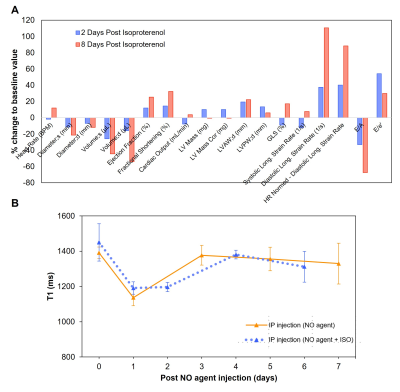 |
Non-invasive magnetic resonance imaging agent for in-vivo nitric
oxide activity detection
Anlan Hong1,2,
Kyle D. W. Vollett1,2,
Aaron M. Troy1,2,
and Hai-Ling Margaret Cheng1,2,3
1Institute of Biomedical Engineering, University of Toronto, Toronto, ON, Canada, 2Translational Biology & Engineering Program, Ted Rogers Centre for Heart Research, Toronto, ON, Canada, 3The Edwards S. Rogers Sr. Department of Electrical & Computer Engineering, University of Toronto, Toronto, ON, Canada Keywords: Inflammation, Preclinical We report a novel nitric oxide (NO)-sensitive MRI contrast agent that holds potential for detecting endogenous NO. Type I collagen and HEK cell lysate showed strong binding with NO agent, while HSA and various ROS/RNS species showed NO agent was only activated by NO. In vivo washout rate was also tested, and NO agent cleared from mice 7 days after intraperitoneal injection. Prolonged enhancement was observed in the heart after isoproterenol injection, which indicated excess NO production and inflammation. The NO agent shows promise as a molecular imaging agent in early detection of inflammatory diseases. |
| 14:49 |
0177.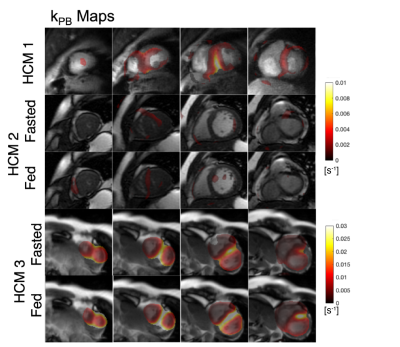 |
Quantification of abnormal metabolism in hypertrophic
cardiomyopathy patients using hyperpolarized [1-13C]-pyruvate
MRI
Avantika Sinha1,
Xiaoxi Liu1,
Shuyu Tang1,2,3,
Nicholas Dwork1,4,
Sanjay Sivalokanathan5,
Jing Liu1,
Robert Bok1,
Karen G Ordovas6,
James Slater1,
Jeremy W Gordon1,
M Roselle Abraham7,
and Peder Eric Zufall Larson1,2 1Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2UC Berkeley-UCSF Graduate Program in Bioengineering, University of California, Berkeley, San Francisco, CA, United States, 3HeartVista, Palo Alto, CA, United States, 4Departments of Bioinformatics and Radiology, University of Colorado School of Medicine, Aurora, CO, United States, 5University of Pennsylvania, Philadelphia, PA, United States, 6University of Washington, Seattle, WA, United States, 7Department of Medicine –Cardiology Division, University of California, San Francisco, San Francisco, CA, United States Keywords: Myocardium, Cardiomyopathy Hypertrophic cardiomyopathy (HCM) is the most common inherited cardiomyopathy with a worldwide prevalence of ~1:250. It is the leading cause of sudden cardiac death often during exercise and in otherwise healthy, young individuals. We applied hyperpolarized [1-13C]-pyruvate MRI for metabolic imaging to 3 patients with HCM using multi-slice, dynamic metabolite-specific imaging with autonomous scanning. We observed focal metabolic abnormalities corresponding to regions of hypertrophy in patients with asymmetric non-obstructive HCM and differences in metabolism measurements compared to healthy volunteers. |
| 14:57 |
0178.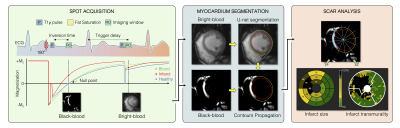 |
Automated detection and quantification of myocardial scar using
AI-powered joint bright- and black-blood LGE imaging
Aurelien Bustin1,2,3,
Indra Ribal1,
Géraldine Montier2,
Jean-David Maes2,
Thibault Boullé2,
Victor de Villedon de Naide1,
Pauline Gut1,3,
Soumaya Sridi2,
Valery Ozenne1,
Marta Nuñez-Garcia1,
Maxime Sermesant1,
Michel Montaudon2,
Gäel Dournes2,
François Laurent2,
Pierre Jaïs1,4,
Matthias Stuber1,3,5,
and Hubert Cochet1,2 1IHU LIRYC, Electrophysiology and Heart Modeling Institute, Université de Bordeaux – INSERM U1045, Bordeaux, France, 2Department of Cardiovascular Imaging, Hôpital Cardiologique du Haut-Lévêque, CHU de Bordeaux, Bordeaux, France, 3Department of Diagnostic and Interventional Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 4Department of Cardiac Electrophysiology, Hôpital Cardiologique du Haut-Lévêque, CHU de Bordeaux, Bordeaux, France, 5CIBM Center for Biomedical Imaging, Lausanne, Switzerland Keywords: Heart, Data Processing Bright-blood late gadolinium-enhanced (LGE) imaging is the current clinical gold standard to assess myocardial scar. However, poor contrast at the blood-scar interface makes scar detection and quantification challenging. Bright- and black-blood LGE imaging technologies have recently enabled more accurate scar detection and localization and have shown promising results for scar quantification. Here we aim to introduce a framework for fully automated scar detection and quantification combining novel joint bright- and black-blood LGE imaging with artificial intelligence-powered analysis. |
| 15:05 |
0179.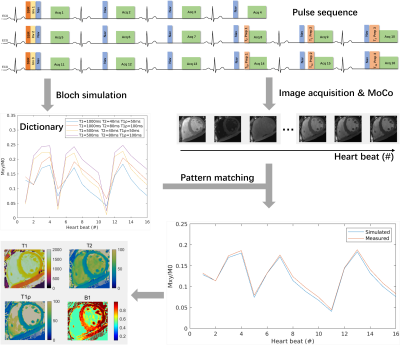 |
Free-Breathing Simultaneous Native Myocardial T1, T2, and T1ρ
Mapping with Cartesian Acquisition and Dictionary Matching
Zhenfeng Lv1,2,
Sha Hua3,
Rui Guo4,
Bowen Shi5,
Peng Hu1,2,
and Haikun Qi1,2 1School of Biomedical Engineering, ShanghaiTech University, Shanghai, China, 2Shanghai Clinical Research and Trial Center, Shanghai, China, 3Department of Cardiovascular Medicine, Ruijin Hospital Lu Wan Branch, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 4School of Medical Technology, Beijing Institute of Technology, Beijing, China, 5iHuman Institute, School of Life Science and Technology, ShanghaiTech University, Shanghai, China Keywords: Myocardium, Tissue Characterization T1 and T2 are well-recognized parameters for detecting various cardiomyopathies, and T1ρ is an endogenous contrast for myocardial fibrosis. Multi-parametric cardiac MRI can provide co-registered parameter maps for comprehensive diagnosis and account for the interparameter dependency for more accurate quantification. Therefore, we propose a free-breathing simultaneous T1, T2, and T1ρ mapping technique with single-shot Cartesian acquisition and dictionary matching for parameter quantification. The phantoms results indicated the good accuracy and insensitiveness to heart rates of the proposed technique. Using prospective through-plane and retrospective in-plane motion correction, the proposed method generated similar in vivo mapping quality to breath-hold mapping methods. |
| 15:13 |
0180.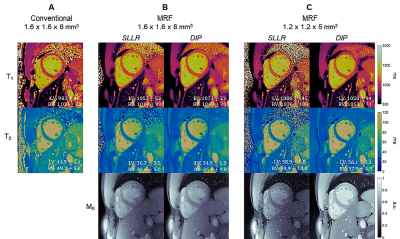 |
High-Resolution Right Ventricular T1, T2, and M0 Mapping Using
MR Fingerprinting with a Deep Image Prior Reconstruction
Jesse Ian Hamilton1,2,
Imran Rashid3,4,
Sanjay Rajagopalan3,4,
and Nicole Seiberlich1,2 1Radiology, University of Michigan, Ann Arbor, MI, United States, 2Biomedical Engineering, University of Michigan, Ann Arbor, MI, United States, 3Harrington Heart and Vascular Institute, University Hospitals Cleveland Medical Center, Cleveland, OH, United States, 4Medicine, Case Western Reserve University, Cleveland, OH, United States Keywords: Myocardium, MR Fingerprinting, Machine Learning/Artificial Intelligence This work proposes a high-resolution (1.2x1.2x5 mm3) MR Fingerprinting method for T1, T2, and M0 mapping of both left and right ventricles during a breathhold, with validation in 12 healthy subjects at 1.5T. A key component is the use of a deep image prior reconstruction for reducing noise and undersampling artifacts despite the decreased SNR when moving to higher resolution. Comparable myocardial relaxation times were measured in both ventricles (LV T1 1061+/-22ms, T2 42.2+/-3.4ms; RV T1 1062+/-29ms, T2 42.7+/-2.5ms). Higher-resolution MRF yielded slightly smaller intersubject standard deviation (SD) but larger intrasubject SD compared to a lower-resolution (1.6x1.6x8 mm3) MRF acquisition. |
| 15:21 |
0181.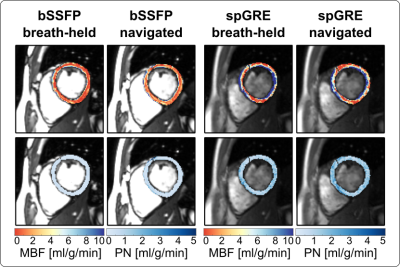 |
Respiratory navigated free-breathing myocardial arterial spin
labeling (ASL) with phase sensitive reconstruction
Masa Bozic-Iven1,2,
Stanislas Rapacchi3,
Qian Tao2,
Lothar R. Schad1,
and Sebastian Weingärtner2
1Heidelberg University, Mannheim, Germany, 2Delft University of Technology, Delft, Netherlands, 3University Aix-Marseille, Marseille, France Keywords: Myocardium, Arterial spin labelling Myocardial arterial spin labeling (myoASL) holds promise for needle-free myocardial blood flow (MBF) quantification but requires tedious averaging over multiple breath-holds. Here, free-breathing myoASL was implemented with dual-navigator gating, both with bSSFP and spoiled GRE readout. Images were processed using individual blood T1 and inversion time correction as well as a phase-sensitive (PS) image reconstruction. Phantom results showed PS reconstruction to reduce MBF variations for short RR and T1 values. Perfusion values were comparable with breath-held myoASL and on par with the literature. Ultimately, this can enable faster myoASL acquisitions with improved patient comfort. |
| 15:29 |
0182.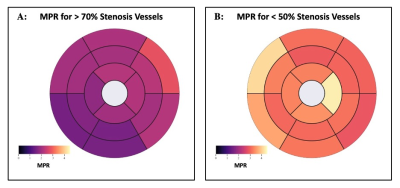 |
Quantitative Perfusion with Rate-Pressure Product Corrections in
Heart Transplant Patients with Cardiac Allograft Vasculopathy
Jay Bharatsingh Bisen1,
Sandra Quinn1,
Ozden Kilinc1,
Havisha Pedamallu1,
Kelvin Chow2,3,
Rachel Davids2,3,
Daniel C Lee4,
Daniel Kim1,
Richard L Weinberg4,
James Carr1,
Michael Markl1,
Bradley D Allen1,
and Ryan Avery1
1Radiology, Northwestern University Feinberg School of Medicine, Chicago, IL, United States, 2Radiology, Northwestern University, Chicago, IL, United States, 3Siemens Healthcare, Chicago, IL, United States, 4Cardiology, Northwestern University Feinberg School of Medicine, Chicago, IL, United States Keywords: Vessels, Quantitative Imaging, Quantitative Perfusion, Heart Transplant, Coronary Allograph Vasculopathy Cardiac Allograft Vasculopathy (CAV) is a form of accelerated intimal hyperplasia in heart transplant patients that limits long-term survival. Quantitative perfusion is a novel MR imaging technique that is useful in determining myocardial blood flow (MBF) and myocardial perfusion reserve (MPR) in CAV patients. After Rate-Pressure Product MBF corrections, Quantitative perfusion has shown that vessels with severe CAV (>70% stenosis) have significantly decreased MPR driven primarily by increased resting MBF. Quantitative perfusion may improve non-invasive monitoring of CAV status in heart transplant patients. |
| 15:37 |
0183.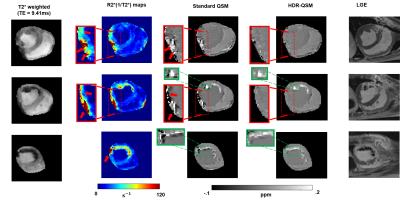 |
Free-breathing, Whole-heart, High-dynamic-range Quantitative
Susceptibility Mapping (HDR-QSM) for Imaging Hemorrhagic
Infarctions
Yuheng Huang1,2,
Xingmin Guan1,
Xinheng Zhang1,2,
Liqi(Richard) Tang 1,
Xiaoming Bi3,
Fei Han3,
HsuLei Lee4,
Hui Han4,
Anthony Christodoulou4,
Debiao Li4,
Rohan Dharmakumar1,
and Hsin-Jung Yang4 1krannert cardiovascular research center, Indiana University school of medicine, Indianapolis, IN, United States, 2Bioengineering, UCLA, LA, CA, United States, 3Siemens Healthineers, Malvern, PA, United States, 4Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States Keywords: Myocardium, Cardiovascular, hemorrhagic reperfusion injury myocardial infarction Accurately detecting and quantifying intramyocardial hemorrhage(IMH) is critical for patient management. QSM has evolved into the standard method for iron imaging. However, obtaining cardiac QSM for IMH evaluation is difficult due to well-known technical challenges. Here, we developed a motion-robust 3D multi-echo GRE technique and combined them with a high-dynamic-range QSM algorithm to derive reliable QSM maps in IMH hearts. We tested and validated it in phantom, ex-vivo, and in-vivo IMH hearts in animal models. We demonstrated that the proposed method could accurately detect IMH and reliably quantify iron concentration with a free-breathing scan under 6 minutes. |
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.