Oral Session
CEST & MT
ISMRM & ISMRT Annual Meeting & Exhibition • 03-08 June 2023 • Toronto, ON, Canada

13:30 |
0899.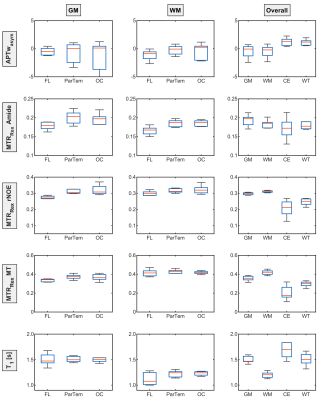 |
Relaxation-compensated CEST-MRI at 3 T exhibits regional
differences in gray matter and white matter contrasts of glioma
patients
Florian Kroh1,2,
Philip S. Boyd1,
Nikolaus von Knebel Doeberitz3,
Heinz-Peter Schlemmer3,4,
Mark E. Ladd1,2,4,
Peter Bachert1,2,
Daniel Paech3,5,
and Andreas Korzowski1
1Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Faculty of Physics and Astronomy, University of Heidelberg, Heidelberg, Germany, 3Division of Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 4Faculty of Medicine, University of Heidelberg, Heidelberg, Germany, 5Division of Neuroradiology, University Hospital Bonn, Bonn, Germany Keywords: CEST & MT, Brain In this study, the APT-weighted (APTwasym) and relaxation-compensated CEST-MRI contrasts of 9 postoperative glioma patients, were analyzed for potential regional differences in gray and white matter. The purpose of this analysis was to identify the influence of the location within the brain on CEST contrasts of tumorous and healthy control tissue, respectively. For relaxation-compensated CEST (MTRRex), (i) differences between gray and white matter and also (ii) regional differences with a decreased contrast inside the frontal lobe were found. On the contrary, the APTwasym was found to be independent of (i) location and (ii) comparable for gray and white matter. |
| 13:38 |
0900.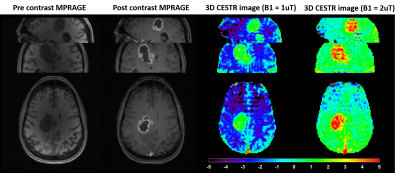 |
Highly Accelerated 3D EPI CEST Imaging using Unevenly Segmented
RF Irradiation with Temporal Random Walk Sampling Pattern in a
Brain Tumor Patient
Hahnsung Kim1,2,
Suhyung Park3,4,
Ranliang Hu2,
Kimberly B Hoang5,
and Phillip Zhe Sun1,2
1Emory National Primate Research Center, Emory University, Atlanta, GA, United States, 2Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, GA, United States, 33Department of Computer Engineering, Chonnam National University, Gwangju, Korea, Republic of, 4Department of ICT Convergence System Engineering, Chonnam National University, Gwangju, Korea, Republic of, 5Department of Neurosurgery, Emory University School of Medicine, Atlanta, GA, United States Keywords: CEST & MT, CEST & MT We proposed a new 3D EPI CEST imaging integrated with unevenly segmented RF irradiation configuration to reduce T1w relaxation-induced signal modulation, thereby allowing for a reliable CEST effect over the whole volume. In addition, a temporal random walk with variable density (VD) CAIPI undersampling is incorporated into segmented 3D EPI for optimized random encoding in CEST imaging. The proposed pulse sequence and reconstruction framework were validated on the phantom and tumor patient. |
13:46 |
0901.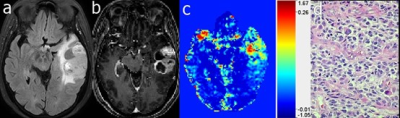 |
Dynamic glucose enhanced MRI of gliomas: a preliminary clinical
application.
Jianhua Mo1,
Xiang Xu2,3,
Andong Ma1,
Mingjun Lu1,
Xianlong Wang1,
Qihong Rui1,
Jianbin Zhu1,
Haitao Wen1,
Genyun Lin1,
Chen Zhao4,
Linda Knutsson3,5,6,
Peter van Zijl3,6,
and Zhibo Wen1
1Department of Radiology, Zhujiang Hospital, Southern Medical University, Guangzhou,Guangdong, China, 2BioMedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4Philips Healthcare, Guangzhou, China, 5Department of Medical Radiation Physics, Lund University, Lund, Sweden, 6F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States Keywords: CEST & MT, Contrast Agent, glucose In this study, we explored the feasibility of dynamic glucose enhanced (DGE) MRI technology in the clinical application of gliomas. 20 glioma patients underwent pre-operative DGE-MRIs before clinical intervention. We observed a significant increase in DGE area under the uptake curve (AUC) signal in tumors compared to the white matter. In some cases, we found enhancement in DGE MRI in histopathological confirmed tumor region that was not enhanced by Gd T1w MRI. These findings provide a new perspective for the further exploration and analysis of the D-glucose delivery, uptake and metabolism in brain tumors. |
| 13:54 |
0902.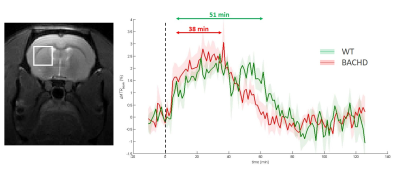 |
Identification of energy metabolism remodeling in a rat model of
Huntington’s disease using multi-metabolic CEST imaging
Yohann Mathieu-Daudé1,
Jérémy Pépin1,
Jean-Baptiste Pérot1,
Cécile Maguin1,
and Julien Flament1
1Université Paris-Saclay, CEA, CNRS, MIRCen, Laboratoire des Maladies Neurodégénératives, Fontenay-aux-Roses, France Keywords: CEST & MT, CEST & MT, gluCEST and glucoCEST Huntington’s disease (HD) is an inherited neurodegenerative disease characterized by motor, cognitive and psychiatric symptoms. As glutamate has been shown to be a potential biomarker of neurodegenerative diseases, we used Chemical Exchange Saturation Transfer imaging of glutamate (gluCEST) to map cerebral glutamate distribution in a rat model of HD. Modification of glutamate levels observed at 12 months were preceded by decrease of lactate concentration and reduced glycolytic metabolism measured glucoCEST at 4 months, suggesting early remodeling of energy metabolism during asymptomatic stage. |
| 14:02 |
0903.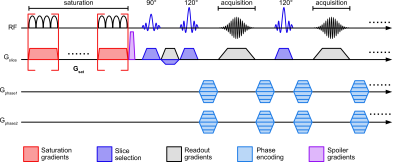 |
Ultrafast Z-spectroscopic Imaging in vivo at 3T using
through-slice spectral encoding (TS-UFZ)
Chongxue Bie1,2,3,
Peter C. M. van Zijl1,2,
Deng Mao1,4,
and Nirbhay N. Yadav1,2
1F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, BALTIMORE, MD, United States, 2The Russell H. Morgan Department of Radiology, The Johns Hopkins University School of Medicine, BALTIMORE, MD, United States, 3Department of Information Science and Technology, Northwest University, Xi’an, China, 4Philips Healthcare, Baltimore, MD, United States Keywords: CEST & MT, CEST & MT Acquisition of high-resolution Z-spectra requires excessive scan times. Ultrafast Z-spectroscopy (UFZ) can obtain whole Z-spectra within one acquisition by encoding the Z-spectral dimension spatially via a gradient applied concurrently with the saturation RF pulse, significantly reducing the scanning time. Still, UFZ has had limited success in vivo due to tissue heterogeneity. Here, we developed a TS-UFZ imaging approach where both saturation gradient and its readout were applied in the slice direction, where there is minimal heterogeneity. Results show that TS-UFZ allows high spectral- and spatial-resolution imaging of CEST signals in phantoms and human brain at 3T. |
| 14:10 |
0904.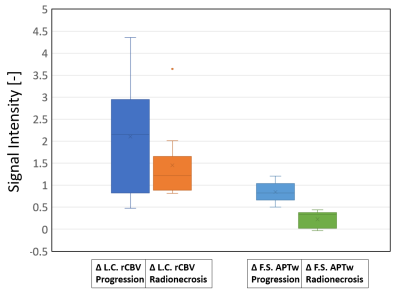 |
Fluid-Suppressed APTw is more accurate than Leakage-Corrected
rCBV imaging in the distinction between tumor progression and
radionecrosis
Lucia Nichelli1,2,
Christos Papageorgakis3,
Mehdi Bensemain4,
Julian Jacob2,5,
Charles Valery6,
Patrick Liebig7,
Moritz Zaiss8,
Stéphane Lehéricy1,2,
and Stefano Casagranda3
1Department of Neurosurgery, Assistance Publique-Hôpitaux de Paris, Groupe Hospitalier Pitié-Salpêtrière-Charles-Foix, Paris, France, 2Sorbonne University, ICM, Paris, France, 3Department of R&D Advanced Applications, Olea Medical, La Ciotat, France, 4Department of Radiology, Nancy Regional University Hospital Centre, Nancy, France, 5Department of Radiation-Oncology, Assistance Publique-Hôpitaux de Paris, Groupe Hospitalier Pitié- Salpêtrière-Charles-Foix, Paris, France, 6Department of Neurosurgery, Assistance Publique-Hôpitaux de Paris, Groupe Hospitalier Pitié- Salpêtrière-Charles-Foix, Paris, France, 7Siemens Healthcare GmbH, Erlangen, Germany, 8Department of Neuroradiology, Friedrich-Alexander Universität Erlangen-Nürnberg (FAU), Erlangen, Germany Keywords: CEST & MT, Tumor, APTw Imaging, Perfusion, Metastases, Radionecrosis, Tumor Progression The distinction between radionecrosis and tumor recurrence is a common diagnostic dilemma, as current advanced multiparametric MRI protocols lack on accuracy. Fluid-Suppressed Amide Proton Transfer weighted (APTw) imaging has strong potentials in brain tumor post-therapeutic assessment. In this study we compare at 3T the diagnostic accuracy of Fluid Suppressed APTw with the most used advanced technique, i.e. the Leakage-Corrected relative Cerebral Blood Volume imaging obtained by DSC perfusion in 22 pre-irradiated metastases. Results show that Fluid-Suppressed APTw metrics can clearly make a distinction between these two pathologies, in contrast to Leakage-Corrected rCBV contrast. |
| 14:18 |
0905.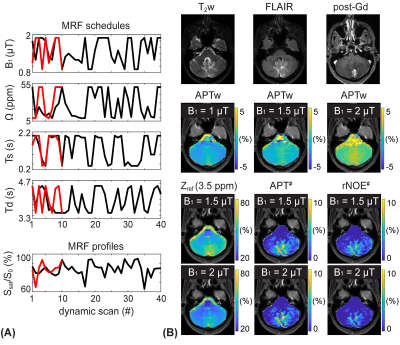 |
Unraveling contributions to the saturated signal at 3.5 ppm in
the Z-spectrum of human brain tumors
Hye-Young Heo1,
Munendra Singh1,
Shanshan Jiang1,
and Jinyuan Zhou1
1Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University, Baltimore, MD, United States Keywords: CEST & MT, CEST & MT Increased cytosolic mobile protein content in gliomas causes amide proton transfer (APT) hyperintensity. However, most current APT imaging protocols acquire APT-weighted images that reflect multiple contributions, including residual direct water saturation (or relaxation), semisolid macromolecular magnetization transfer contrast (MTC) asymmetry, and nuclear Overhauser enhancement (NOE) effects, thus limiting the assessment of clean APT. Herein, we separated water, MTC, and APT signal components from RF saturated signals using an MR fingerprinting sequence and evaluated the contributions to the saturation signal at 3.5 ppm in the Z-spectrum of brain tumors. |
| 14:26 |
0906.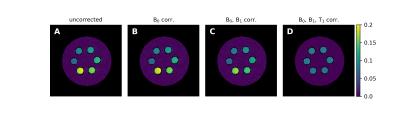 |
An Analytic Solution for the Modified WASABI Method: Application
to Simultaneous B0, B1 and T1 Mapping and Correction of CEST MRI
Patrick Schuenke1,
Felix Frederik Zimmermann1,
Kerstin Kaspar1,
Moritz Zaiss2,
and Christoph Kolbitsch1
1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany, 2Institute for Neuroradiology, University Hospital Erlangen, Friedrich-Alexander Universität Erlangen-Nürnberg, Erlangen, Germany Keywords: CEST & MT, Data Processing CEST MRI provides a contrast sensitive to exchange processes between solute and water protons that was proven to add value in clinical MRI. However, the contrast is susceptible to B0- and B1 field inhomogeneities as well as the T1 relaxation time. Here we present an analytical solution for a modified WASABI method that enables the quantitative mapping of all three parameters simultaneously from a single CEST-like MRI scan. We show that the generated parameter maps and reference maps match well and demonstrate their applicability for the B0, B1 and T1 correction of CEST MRI data. |
| 14:34 |
0907.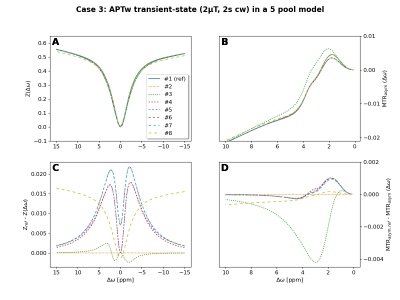 |
Validate your CEST simulation!
Patrick Schuenke1,
Kai Herz2,3,
Zhongliang Zu4,
Nirbhay Yadav5,6,
Qing Zeng5,6,
Markus Huemer7,
Rudolf Stollberger7,
Jiadi Xu5,6,
Kexin Wang5,8,
Feriel Romdhane9,
Dario Livio Longo9,
Or Perlman10,
Peter C.M. van Zijl5,8,
and Moritz Zaiss11
1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany, 2Magnetic Resonance Center, Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 3Department of Biomedical Magnetic Resonance, University of Tuebingen, Tuebingen, Germany, 4Department of Radiology, Vanderbilt University Medical Center, Nashville, TN, United States, 5FM Kirby Research Center, Kennedy Krieger Institute, Baltimore, MD, United States, 6Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 7Institut of Bioimaging, Graz University of Technology, Graz, Austria, 8Department of Radiology, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 9Institute of Biostructures and Bioimaging (IBB), National Research Council of Italy (CNR), Torino, Italy, 10Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 11Institute for Neuroradiology, University Hospital Erlangen, Friedrich-Alexander Universität Erlangen-Nürnberg, Erlangen, Germany Keywords: CEST & MT, Simulations, validation, comparison Quantitative CEST approaches can provide access to the exchange rate and solute concentration of exchange processes of interest. However, differences in the implementation of underlying Bloch-McConnell simulations will lead to differences in the determined quantitative parameters. Thus, we initiated a platform to compare and validate the CEST simulations from various CEST research groups. In this first comparison of continuous-wave saturation schemes, up to 5 different results for 8 compared simulations were observed. The project is still open for participation and we invite everbody to join the comparison and our planned discussions to finally realize a consensus on CEST simulations. |
| 14:42 |
0908.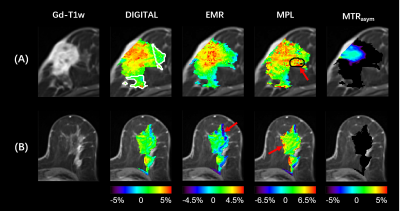 |
Removal of lipid artifacts in CEST data using differential
analysis with fitted magnetization transfer and lipid signals
(DIGITAL)
Zhechuan Dai1,
Xingwang Yong1,
Jing Zhang2,
Xiaoxia Wang2,
Qing Li3,
Yi Sun3,
Ying Cao4,
Jiuquan Zhang2,
and Yi Zhang1
1Key Laboratory for Biomedical Engineering of Ministry of Education, Department of Biomedical Engineering, College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, China, 2Department of Radiology, Chongqing University Cancer Hospital, Chongqing, China, 3MR Collaborations, Siemens Healthineers Ltd., Shanghai, China, 4School of Medicine, Chongqing University, Chongqing, China Keywords: CEST & MT, CEST & MT Chemical exchange saturation transfer (CEST) imaging provides important molecular information that can reflect changes in various pathologies. However, applying CEST to lipid-rich organs, such as the breast, is technically challenging, where the lipid artifacts can grossly affect the CEST signal. In this study, we propose a novel differential analysis method with fitted magnetization transfer and lipid contributions (DIGITAL) to remove the lipid artifacts without changing the acquisition sequence. The DIGITAL method was validated on breast cancer patients, and yielded fewer lipid artifacts and better image smoothness than previous analysis-based methods. |
| 14:50 |
0909.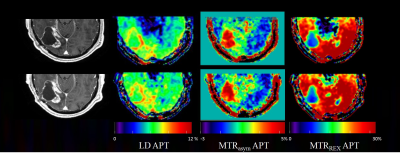 |
Can APT-weighted CEST MRI provide robust measurement at 3T? A
reproducibility study in healthy brain and tumor across sessions
and scanners
Yulun Wu1,2,
Tobias C. Wood3,
Sophie H.A.E. Derks1,4,
Ilanah J. Pruis1,
Sebastian van der Voort1,5,
Sophie E.M. Veldhuijzen van Zanten1,
Marion Smits1,2,5,
and Esther A.H. Warnert1,2 1Department of Radiology & Nuclear Medicine, Erasmus MC, Rotterdam, Netherlands, 2Brain Tumour Centre, Erasmus MC Cancer Institute, Rotterdam, Netherlands, 3Centre for Neuroimaging Science, King's College London, London, United Kingdom, 4Department of Medical Oncology, Erasmus MC, Rotterdam, Netherlands, 5Medical Delta, Delft, Netherlands Keywords: CEST & MT, CEST & MT, Amide, reproducibility, brain tumor The goal of this study was to investigate whether APT-weighted CEST imaging can provide reproducible measurements across scan sessions and scanners. Reproducibility of APT-weighted imaging in healthy brain tissue and tumors was evaluated for three CEST metrics: Lorentzian Difference (LD), magnetization transfer ratio asymmetry (MTRasym), and relaxation-compensated inverse magnetization transfer ratio (MTRREX). |
| 14:58 |
0910.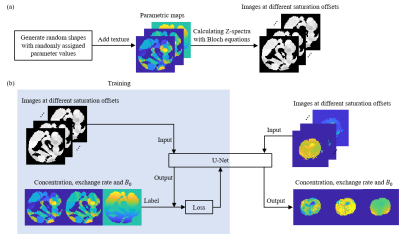 |
Quantification of NOE effect and its application on brain tumor
detection
Jingyi Yu1,
Jian Wu1,
Xinli Lan1,
Yonggui Yang2,
Zhigang Wu3,
Congbo Cai1,
and Shuhui Cai1
1Department of Electronic Science, Xiamen University, Xiamen, China, 2Department of Radiology, The Second Affiliated Hospital of Xiamen Medical College, Xiamen, China, 3MSC Clinical & Technical Solutions, Philips Healthcare, Shenzhen, China Keywords: CEST & MT, Machine Learning/Artificial Intelligence Chemical exchange saturation transfer (CEST) quantification is mostly based on pixel-wise fitting of Z-spectra, which is time-consuming and noise-sensitive. Herein, we propose an approach to quantify CEST based on U-Net. The proposed method can simultaneously quantify the concentration and the exchange rate of nuclear Overhauser enhancement (NOE), together with the B0 map. The results of a simulation sample and a rat C6 glioma model suggest that the quantification of NOE effect with U-Net is accurate, precise and fast. |
| 15:06 |
0911.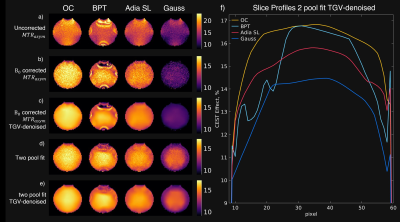 |
B0-Robustness and Exchange Weighting of Optimal Control Pulses
Compared to State of the Art Pulses for CEST Imaging
Clemens Stilianu1,
Markus Huemer1,
Christina Graf1,
Clemens Diwoky2,
Armin Rund3,
Moritz Zaiss4,5,
and Rudolf Stollberger1
1Institute of Biomedical Imaging, Graz University of Technology, Graz, Austria, 2Institute of Molecular Biosciences, University of Graz, Graz, Austria, 3Institute for Mathematics and Scientific Computing, University of Graz, Graz, Austria, 4Department High-field Magnetic Resonance, Max Planck Institute Tübingen, Tübingen, Germany, 5Institute of Neuroradiology, Friedrich-Alexander University Erlangen-Nürnberg, Erlangen, Germany Keywords: CEST & MT, CEST & MT, Spin lock, CESL The exchange weighting and robustness to B0-inhomogeneities are essential properties for accurate CEST-MRI saturation measurements. Therefore, we investigated these properties for a new numerical optimized saturation pulse train and for state-of-the-art saturation pulse strategies in simulation and phantom measurements. We have found that the optimized pulse train generates superior CEST contrast and has very high stability against B0-inhomogenities while maintaining the natural shape at the water peak which improves CEST-spectrum fitting and B0-correction. |
| 15:14 |
0912.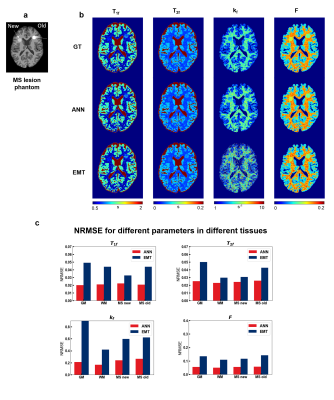 |
Improving bSSFP-based quantitative magnetization transfer
imaging with MR physics-informed artificial neural network
Huan Minh Luu1 and
Sung-Hong Park1
1Bio and Brain Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea, Republic of Keywords: CEST & MT, Quantitative Imaging Most quantitative magnetization transfer imaging (qMT) protocols require additional T1 mapping scan. A recent on-resonance multiple phase-cycle bSSFP method was proposed for qMT that obviates the necessity for T1 mapping, but the fitting results were suboptimal. In this study, we proposed a physics-informed artificial neural network (ANN) to improve the fitting of this method. By using the MR signal model to generate the training data and regularize the network, no in-vivo data acquisition was necessary. Experiments on digital phantom and in-vivo data demonstrated improvement over previous method and better resilience against measurement noise. |
| 15:22 |
0913.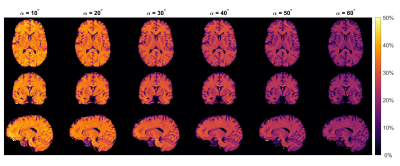 |
MT imaging at 0.55T with GRE and bSSFP
David Leitão1,
Daniel West1,
Raphael Tomi-Tricot2,3,
Jo Hajnal1,3,
Tobias C Wood4,
and Shaihan Malik1,3
1Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare, Frimley, United Kingdom, 3Centre for the Developing Brain, King's College London, London, United Kingdom, 4Department of Neuroimaging, King's College London, London, United Kingdom Keywords: Magnetization transfer, Magnetization transfer, Low-Field MRI We used multiband pulses to obtain Magnetization Transfer (MT) weighted images at 0.55T in a time-efficient manner. White Matter (WM)/Grey Matter (GM) contrast is compared across several flip-angles for both GRE and bSSFP sequences. GRE showed larger MTR WM/GM difference but after accounting for signal-to-noise ratio bSSFP had 50% better contrast-to-noise ratio. |
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.