Oral Session
X-Nuclei MR
ISMRM & ISMRT Annual Meeting & Exhibition • 03-08 June 2023 • Toronto, ON, Canada

08:15 |
0009.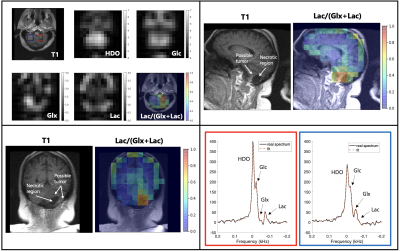 |
Deuterium Metabolic Imaging (DMI) for 3D Mapping of Glucose
Metabolism in Humans with Central Nervous System Lesions at 3T
Philip M. Adamson1,
Keshav Datta2,
Ron Watkins2,
Lawrence Recht3,
Ralph Hurd2,
and Daniel Spielman2 1Department of Electrical Engineering, Stanford University, Palo Alto, CA, United States, 2Department of Radiology, Stanford University, Palo Alto, CA, United States, 3Department of Neurology, Stanford University, Palo Alto, CA, United States Keywords: Deuterium, Deuterium DMI is an emerging modality for investigating glucose metabolism in vivo with application for assessing the Warburg effect in tumors. Although high-field systems, e.g. 7T, provide maximal signal-to-noise ratio (SNR), implementation on widely available 3T scanners could have immediate clinical impact. Here we explore the potential of 3T DMI using a birdcage 2H RF coil in two healthy volunteers and three patients with CNS lesions of varying pathology. Results from these experiments demonstrate the potential to examine the Warburg effect in CNS lesions with DMI at 3T and provide critical data needed to explore DMI SNR and spatial resolution limits. |
08:23 |
0010.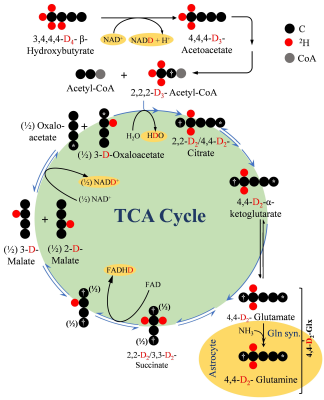 |
In Vivo Assessment of β-Hydroxybutyrate Metabolism in Mouse
Brain Using Deuterium (2H) Magnetic Resonance Spectroscopy
Narayan Datt Soni1,
Anshuman Swain1,
Paul Jacobs1,
Halvor Juul1,
Ryan Armbruster1,
Ravi Prakash Reddy Nanga1,
Kavindra Nath1,
Corinde Wiers2,
John Detre3,
and Ravinder Reddy1
1Department of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 2Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 3Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States Keywords: Spectroscopy, Deuterium, , 2H-MRS, Brain, TCA, Metabolism Impaired neurometabolism is often attributed to altered TCA flux (VTCA). Since, β-hydroxybutyrate metabolism bypasses glycolytic flux, it can be better used to monitor VTCA. In the current study, [3,4,4,4]-2H4-β-hydroxybutyrate was infused in 6-month-old mice, and the level of [4,4]-2H2-Glx was monitored using 2H-MRS. A kinetic model was fitted to determine the rate of BHB metabolism (CMRBHB) and VTCA. Glx labeling followed a sigmoidal curve to reach a quasi-steady state concentration (~1.6±0.1 mM) in 30 minutes. CMRBHB and VTCA were determined to be 0.054±0.004 and 0.12±0.01 µM/g/min. In conclusion, this method can be used to monitor neurometabolism in health and disease. |
08:31 |
0011.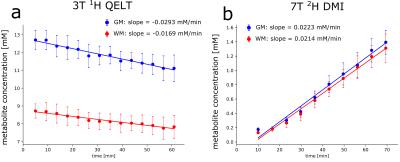 |
Reproducibility of non-invasive 3D imaging of glucose downstream
metabolism using deuterium labeling: indirect 1H QELT at 3T vs
direct 2H DMI at 7T
Fabian Niess1,
Lukas Hingerl1,
Bernhard Strasser1,
Petr Bednarik2,3,
Dario Goranovic1,
Eva Niess1,
Stanislav Motyka1,
Gilbert Hangel1,4,
Martin Krssak5,
Benjamin Spurny-Dworak6,
Thomas Scherer5,
Rupert Lanzenberger6,
and Wolfgang Bogner1
1Department of Biomedical Imaging and Image-Guided Therapy, Medical University of Vienna, Vienna, Austria, 2Centre for Functional and Diagnostic Imaging and Research, Danish Research Centre for Magnetic Resonance, Copenhagen, Denmark, 3Department of Radiology, University Hospital Amager and Hvidovre, Copenhagen, Denmark, 4Department of Neurosurgery, Medical University of Vienna, Vienna, Austria, 5Department of Medicine III, Division of Endocrinology and Metabolism, Medical University of Vienna, Vienna, Austria, 6Department of Psychiatry and Psychotherapy, Comprehensive Center for Clinical Neurosciences and Mental Health (C3NMH), Medical University of Vienna, Vienna, Austria Keywords: Deuterium, Brain Deuterium metabolic imaging (DMI) and quantitative exchange label turnover (QELT) are emerging MR techniques to non-invasively map brain glucose uptake and downstream metabolism using safe and affordable deuterium labeled glucose as tracer. This work compares dynamically detected deuterium labeled Glutamate+Glutamine time courses between three-dimensional 3T-1H QELT-MRSI and 7T-2H-DMI using quantification of concentration estimates. Comparable enrichment of deuterated Glx could be observed in gray matter (1.59±0.24mM vs. 1.39±0.17mM) while differences were found in WM (0.89±0.32 mM vs. 1.31±0.2 mM) presumably due to partial volume contamination. Results showed a successful translation of QELT to clinical 3T. |
| 08:39 |
0012.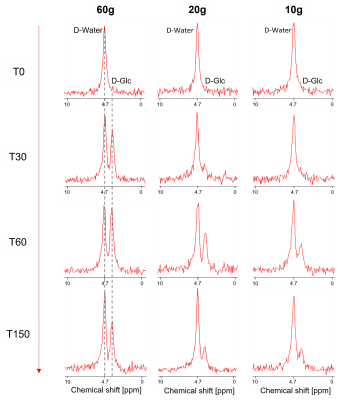 |
Evaluation of minimal dosage of deuterated glucose for mapping
of hepatic metabolism by DMI and natural abundance carbon-13
spectroscopy at 7 T
Simone Poli1,2,
Ahmed F. Emara3,
Edona Ballabani3,
Naomi F. Lange4,5,
Andreas Melmer3,
David Herzig3,
Luc Tappy3,
Lia Bally3,
and Roland Kreis1,2 1Magnetic Resonance Methodology, Institute of Diagnostic and Interventional Neuroradiology, University of Bern, Bern, Switzerland, 2Translational Imaging Center, Sitem-insel, Bern, Switzerland, 3Department of Diabetes, Endocrinology, Nutritional Medicine and Metabolism UDEM, Inselspital, University Hospital Bern, Bern, Switzerland, 4Department of Visceral Surgery and Medicine, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland, 5Graduate School for Health Sciences, University of Bern, Bern, Switzerland Keywords: Deuterium, Metabolism We present an estimation of the minimal dose for D-glucose needed for evaluation of hepatic glucose dynamics by deuterium metabolic imaging (DMI) and investigate whether a subsequent change in hepatic glycogen content is observable by 13C-MRS at 7 T. Six healthy subjects received an oral glucose load of 60g, 20g or 10g and were examined with interleaved 2H-MRSI/13C-MRS scans for 150 min. Results suggest the feasibility of reducing D-glucose loads to as low as 10g per subject for hepatic DMI with several benefits ‑ including cost, but precision of determination of changes in hepatic glycogen content is substantially reduced. |
| 08:47 |
0013.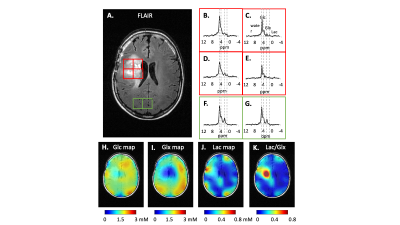 |
Expanding interleaved MRI-DMI to 3D SWI (Susceptibility Weighted
Imaging) : A truncated acquisition approach
Yanning Liu1,
Zachary Corbin1,
Robert K. Fulbright1,
Terry W. Nixon1,
Scott McIntyre1,
Henk De Feyter1,
and Robin A. de Graaf1
1Yale University, New Haven, CT, United States Keywords: Deuterium, Deuterium, DMI Interleaved MRI-DMI (Deuterium Metabolic Imaging) is a transformative approach to obtain multi-contrast anatomic MRI and metabolic information in parallel with time efficiency. In this work, we expand the toolkit of interleaving 2H DMI with 1H MRI, by using a newly developed interleaved 3D SWI-DMI sequence that relies on acquiring a truncated 2H signal. Acknowledging the limitations of fitting truncated 2H signals independently, we propose a method to combine truncated FID with fully-sampled acquisitions to improve DMI sensitivity. The updated interleaved MRI-DMI protocol with 3D SWI-DMI was demonstrated on a patient with a brain tumor. |
| 08:55 |
0014.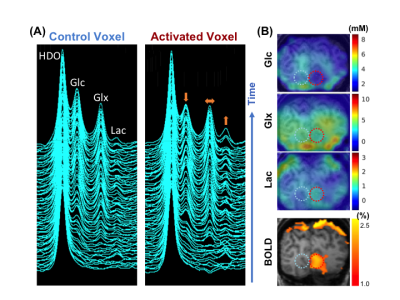 |
Functional Study of Glucose Consumption, TCA Cycle Activity and
Lactate Production in Healthy Human Brain Using Dynamic 2H MRS
Imaging at 7T
Xiao-Hong Zhu1,
Wei Zhu1,
Hannes M Wiesner1,
Yudu Li2,3,
Tao Wang1,
Zhi-Pei Liang2,4,
and Wei Chen1
1Center for Magnetic Resonance Research, Department of Radiology, University of Minnesota, Minneapolis, MN, United States, 2Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 3National Center for Supercomputing Applications, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 4Department of Electrical and Computer Engineering, University of Illinois at Urbana-Champaign, Urbana, IL, United States Keywords: Deuterium, fMRI, Functional deuterium metabolic imaging The relationship of total glucose consumption, TCA cycle and glycolysis in healthy human brain under resting and activated conditions has not been well studied due to the lack of effective neuroimaging tools. The recently developed dynamic deuterium metabolic imaging method provides a long desired capability for such studies. Here, we present the first functional deuterium imaging study in human brains during hemi-field visual stimulation at 7T. Our results provide new insights into the metabolic shifting between oxidative phosphorylation and aerobic glycolysis from the rest to activated state in supporting high energy demand of evoked activity in human visual cortex. |
| 09:03 |
0015.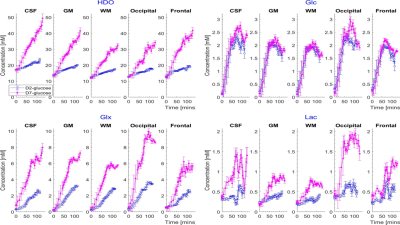 |
Comparison of deuterium metabolic imaging measurements in human
subjects at 7T following [2H2] glucose and [2H7] glucose
ingestion
Daniel Cocking1,2,
Robin Damion1,3,4,
Elizabeth Simpson3,
Paul Greenhaff3,5,6,
Louise Dexter1,2,
Dorothee Auer1,3,4,
and Richard Bowtell1,2,3
1Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom, 2School of Physics and Astronomy, University of Nottingham, Nottingham, United Kingdom, 3NIHR Nottingham Biomedical Research Centre/Nottingham Clinical Research Facilities, Queen's Medical Centre, Nottingham, United Kingdom, 4Radiological Sciences, Division of Clinical Neuroscience, School of Medicine, University of Nottingham, Nottingham, United Kingdom, 5MRC-Versus Arthritis Centre for Muscoskeletal Ageing Research, University of Nottingham, Nottingham, United Kingdom, 6School of Life Sciences, University of Nottingham, Nottingham, United Kingdom Keywords: Deuterium, Deuterium We compared the use of DMI following D2-glucose and D7-glucose ingestion in the investigation of brain metabolism in vivo. Multiple CSI measurements were acquired from five participants over ~180 minutes at 7T following ingestion of each labelled compound. D7-glucose produced significantly larger signals allowing improved mapping of the signals from labelled metabolites. Calculated time-courses of the changes in metabolite concentration in different brain tissues show significantly larger rates of increase of HDO and Glx in all tissues for D7- versus D2-glucose (e.g. in GM HDO rate of increase for D7/D2 = 0.208±0.004/0.051±0.001 mM/min and Glx rate for D7/D2 = 0.059±0.003/0.029±0.001 mM/min). |
| 09:11 |
0016.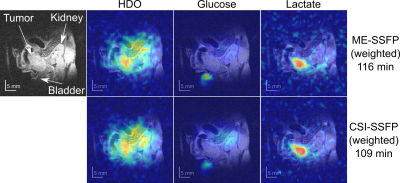 |
Improved Deuterium Metabolic Imaging of Cancer by CSI-SSFP MRSI
Elton Montrazi1,
Dana Peters2,
Keren Sasson1,
Lilach Agemy1,
Avigdor Scherz1,
and Lucio Frydman1
1Weizmann, Rehovot, Israel, 2Yale School of Medicine, New Haven, CT, United States Keywords: Deuterium, Cancer, MRSI Deuterium metabolic imaging (DMI) is a promising tool for studying tumor metabolism. In DMI [6,6’-2H2]-glucose is uptaken by tumors, leading to the formation of HDO and of [3,3’-2H2]-lactate as result of Warburg effect. DMI’s biggest challenge is SNR, consequence of 2H’s low Larmor frequency and the low concentrations of the targets. Depending on the type and size of the tumor, this can bring the key lactate signal below the noise level. This work explores weighted chemical shift imaging (CSI) methodologies for DMI based on SSFP sequences, providing improved lactate sensitivity over recently discussed CSI and multi-echo (ME) SSFP approaches. |
| 09:19 |
0017.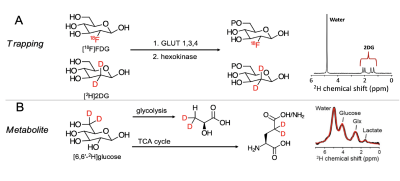 |
Exploring glucose metabolic impairment in Alzheimer’s Disease
with DMI using [6,6′-2H2]-D-glucose and
[2,2’-2H2]-deoxy-D-glucose at 14.1T
Xiao Gao1,
Jeremy Gordon2,
Kai Qiao2,
Hecong Qin2,
David Wilson2,
and Myriam M Chaumeil2
1Physical Therapy, UCSF, San Francisco, CA, United States, 2UCSF, San Francisco, CA, United States Keywords: Deuterium, Alzheimer's Disease We performed a deuterium metabolic imaging (DMI) investigation of two deuterated probes with different metabolic properties: 1) the glucose analog [2,2’-2H2]-deoxy-D-glucose ([2H2]2DG) which gets intracellularly trapped after being phosphorylated by hexokinase; and 2) [6,6′-2H2]-D-glucose ([2H2]Glc) which undergoes full oxidative catabolism. We tested their potential to detect metabolic impairment in a mouse model of Alzheimer’s disease. Our results demonstrated that the accumulation / metabolism of these two glucose derivatives can be detected dynamically in the mouse brain, and that DMI can detect disrupted glucose metabolism in an AD model. |
| 09:27 |
0018.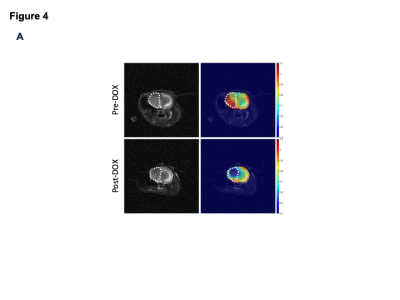 |
Imaging choline phospholipid biosynthesis in gliomas using
deuterium magnetic resonance spectroscopy
Celine Taglang1,
Georgios Batsios1,
Anne-Marie Gillespie1,
and Pavithra Viswanath1
1Radiology and Biomedical imaging, University of California San Francisco, San Francisco, CA, United States Keywords: Deuterium, Cancer Aberrant choline phospholipid biosynthesis is a metabolic hallmark of cancer. Choline kinase α (CKα) is the key enzyme in this pathway. Non-invasive methods of imaging CKα have the potential to report on tumor proliferation. Here, using a combination of RNA interference and doxycycline-inducible gene silencing systems, we show that deuterium magnetic resonance spectroscopy following administration of [2H9]-choline non-invasively reports on CKα activity in patient-derived glioma cells and intracranial tumors. Importantly, [2H9]-choline provides an early readout of response to chemotherapy in mice bearing intracranial gliomas. Our studies identify a novel agent for metabolic imaging of gliomas and their response to therapy. |
| 09:35 |
0019.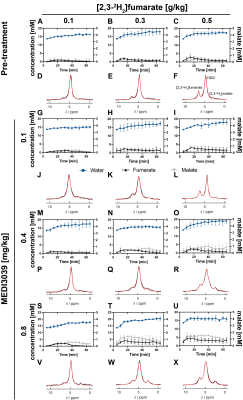 |
Determining the minimum injected [2,3-2H2]fumarate concentration
required for deuterium metabolic imaging of tumor cell death
post-treatment
Friederike Hesse1,2,
Flaviu Bulat1,3,
Felix Kreis1,
and Kevin M. Brindle1,4
1CRUK CI, University of Cambridge, Cambridge, United Kingdom, 2Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 3Department of Chemistry, University of Cambridge, Cambridge, United Kingdom, 4Department of Biochemistry, University of Cambridge, Cambridge, United Kingdom Keywords: Deuterium, Molecular Imaging 2H MRI measurements of labeled malate production following intravenous injection of 2H-labeled fumarate (1 g/kg) have been used to detect tumor cell death post-treatment in pre-clinical tumor models. We show here that much lower concentrations of 2H-labeled fumarate (0.1, 0.3 and 0.5 g/kg) can still generate a significant increase in malate concentration post-treatment, which should facilitate clinical translation. Mice were implanted subcutaneously with a triple-negative breast cancer model (MDA-MB-231) and treated with three different concentrations of a TRAIL-R2 agonist (MEDI3039). The different degrees of cell death obtained were linearly correlated with the malate/fumarate signal ratio and the absolute malate concentration. |
| 09:43 |
0020.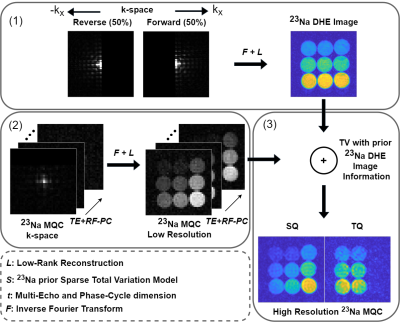 |
Improved 23Na Multi-Quantum Coherences MRI with simultaneous
Cartesian Double-Half-Echo Sodium readouts
Christian Licht1,
Jascha Zapp1,
Mark Bydder2,
Maxime Guye2,
Lothar R. Schad1,
and Stanislas Rapacchi2
1Computer Assisted Clinical Medicine, Heidelberg University, Mannheim, Germany, 2CRMBM, Aix-Marseille Université, Marseille, France Keywords: Non-Proton, Multi-Contrast, Sodium Sodium (23Na) MRI has received increased attention as a potential biomarker for disease states thanks to the advent of high and ultra-high field MRI. One asset from 23Na MRI is the distinction between single and triple quantum signal to provide new insights in tissue characterization. Hence, efficient sequences are needed that enable simultaneous 23Na and 23Na multi-quantum-coherences imaging to fully exploit the signal's potential. Therefore, we propose a new sequence utilizing a double-half-echo 23Na readout. Additionally, we propose to exploit this image further to drive the reconstruction of the low resolution MQC images. |
| 09:51 |
0021.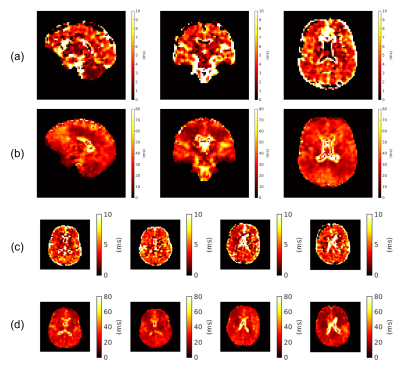 |
Whole-brain bi-exponential 23Na-MRI T2* mapping at 7T with a
32-channel phased array receiver coil
Samuel Rot1,2,
Jon O Cleary3,
Ayse Sila Dokumaci4,5,
Michael Eyre4,5,6,
Philippa Bridgen5,7,
Yasmin Blunck8,9,
Warda Syeda10,
Shaihan J Malik4,5,
Joseph V Hajnal4,5,
Bhavana S Solanky1,11,
Shan-Shan Tang6,
Claudia AM Gandini Wheeler-Kingshott1,12,13,
and David Carmichael4,5
1NMR Research Unit, Queen Square MS Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, Faculty of Brain Sciences, University College London, London, United Kingdom, 2Department of Medical Physics and Biomedical Engineering, University College London, London, United Kingdom, 3Department of Radiology, Imperial College Healthcare NHS Trust, London, United Kingdom, 4Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King’s College London, London, United Kingdom, 5London Collaborative Ultra high field System (LoCUS), London, United Kingdom, 6Children's Neurosciences, Evelina London Children's Hospital at Guy's and St Thomas' NHS Foundation Trust, London, United Kingdom, 7Guys and St Thomas’ NHS Foundation Trust, King's College London, London, United Kingdom, 8Department of Biomedical Engineering, The University of Melbourne, Melbourne, Australia, 9Melbourne Brain Imaging Unit, The University of Melbourne, Melbourne, Australia, 10Melbourne Neuropsychiatry Centre, The University of Melbourne, Parkville, Victoria, Australia, 11Quantitative Imaging Group, Department of Medical Physics and Biomedical Engineering, University College London, London, United Kingdom, 12Department of Brain & Behavioural Sciences, University of Pavia, Pavia, Italy, 13Brain Connectivity Centre Research Department, IRCCS Mondino Foundation, Pavia, Italy Keywords: Non-Proton, Relaxometry, Sodium, T2* mapping In vivo 23Na-MRI benefits greatly from SNR improvements at ultrahigh fields and with multi-channel receivers. Here, we report a pipeline for multi-echo radial imaging of 23Na (MERINA) at 7T, using a 32-channel receiver coil. This involves correction of image artifacts induced by gradient imperfections, followed by channel combination to maintain Rician noise distributions in combined magnitude images. This led to improved T2* mapping with a fixed-component bi-exponential signal model. T2* values (e.g. T2s*=4.6±0.9ms, T2l*=28.3±2.8ms in cerebral white matter) agree with reports in literature. Future work will involve correcting B0 inhomogeneity effects, more sophisticated signal models and exploring potential clinical applications. |
09:59 |
0022.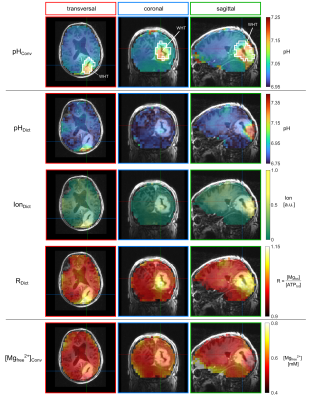 |
Estimation of pH values and magnesium ion content in vivo using
a chemical shift dictionary for 31P MRSI at UHF
Vanessa L. Franke1,2,
Johannes S. Breitling1,
Renate Bangert1,
Philip S. Boyd1,
Nina Weckesser3,
Heinz-Peter Schlemmer3,4,
Daniel Paech3,5,
Mark E. Ladd1,2,4,
Peter Bachert1,2,
and Andreas Korzowski1
1Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Faculty of Physics and Astronomy, University of Heidelberg, Heidelberg, Germany, 3Division of Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 4Faculty of Medicine, University of Heidelberg, Heidelberg, Germany, 5Division of Neuroradiology, University Hospital Bonn, Bonn, Germany Keywords: Spectroscopy, Non-Proton, 31P, phosphorus, pH 31P MRSI enables non-invasive mapping of pH value and magnesium content in vivo, and is conventionally done by calibration equations, like the modified Henderson-Hasselbalch equation, relating 31P chemical shifts to physiological parameters. The reliability of this approach might be hampered when applied to diseased tissue, like cancer, where the chemical conditions are altered. Recently, we proposed a chemical shift dictionary for 31P MRSI to potentially enable a condition-independent estimation of pH value and magnesium content. In this study, the applicability of this approach to in vivo data is tested, and its potential for further research identified. |
| 10:07 |
0023.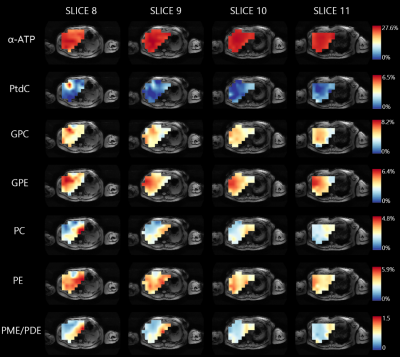 |
Spatial variations of 31P metabolites within the liver measured
with 3D 31P MRSI at 7T
Lieke van den Wildenberg1,
Ayhan Gursan1,
Leonard Seelen1,
Tijl van der Velden1,
Mark Gosselink1,
Martijn Froeling1,
Wybe van der Kemp1,
Dennis Klomp1,
and Jeanine Prompers1
1Center for Image Sciences, University Medical Center Utrecht, Utrecht, Netherlands Keywords: Liver, Spectroscopy 31P MRS can be used in liver disease diagnosis and treatment monitoring. However, it is unclear whether 31P metabolite concentrations differ across the healthy liver, which would be important to take into account. Using an integrated 31P whole-body transmit coil for a 7T MRI scanner in combination with a 16-channel body receive array, we aimed to assess spatial variations of 31P metabolites within the liver in healthy subjects. We showed that there is significant spatial heterogeneity of various 31P metabolites levels within the liver, with marked differences for the PME and PDE metabolites between the left and right lobe. |
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.