Oral Session
Novel Quantitative Imaging Methods: Acquisition
ISMRM & ISMRT Annual Meeting & Exhibition • 03-08 June 2023 • Toronto, ON, Canada

15:45 |
0664.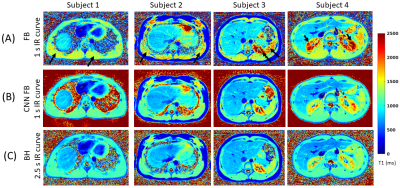 |
T1 mapping of the entire abdomen using a time efficient free
breathing neural network radial Look Locker approach
Eze Ahanonu1,
Kevin Johnson2,
Ute Goerke3,
Brian Toner4,
Vibhas Deshpande5,
Ali Bilgin1,2,6,
and Maria Altbach2,6
1Department of Electrical and Computer Engineering, The University of Arizona, Tucson, AZ, United States, 2Department of Medical Imaging, The University of Arizona, Tucson, AZ, United States, 3Siemens Healthineers, Tucson, AZ, United States, 4Applied Math Program, The University of Arizona, Tucson, AZ, United States, 5Siemens Healthineers, Austin, TX, United States, 6Department of Biomedical Engineering, The University of Arizona, Tucson, AZ, United States Keywords: Quantitative Imaging, Body The interest in developing quantitative metrics in abdominal imaging has grown in recent years. In particular, abdominal T1 mapping plays a role in the characterization of abdominal pathologies. However, current T1 mapping of the abdomen is limited by poor anatomical coverage, long acquisitions related to sufficient sampling of the T1 recovery curve and recovery times, and reduced T1 accuracy secondary to respiratory motion. Here we present a novel approach for free-breathing T1 mapping of the abdomen, which leverages the undersampling robustness of radial MRI and combines fast data acquisition with deep learning for accurate and efficient abdominal T1 mapping. |
| 15:53 |
0665.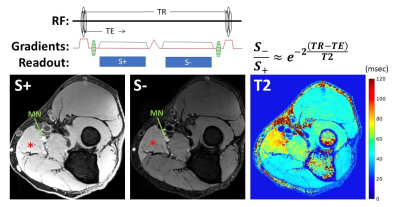 |
Quantitative 3D DESS T2 mapping with Deep Learning
Reconstruction for Magnetic Resonance Neurography
Gracyn Campbell1,
Darryl B. Sneag1,
Qian Li1,
and Ek Tsoon Tan1
1Hospital for Special Surgery, New York, NY, United States Keywords: Quantitative Imaging, Nerves, Deep Learning Conventional, quantitative T2 mapping for MR neurography may depict peripheral neuropathy related changes but has insufficient spatial resolution within acceptable acquisition times (<6 min.) to mitigate motion. Alternatively, dual-echo steady-state (DESS) can simultaneously provide high resolution 3D qualitative anatomical data and quantitative T2 maps for characterizing both nerve and muscle within this targeted acquisition window. Analysis of subjects with peripheral neuropathy in the elbow/forearm region showed that DESS-T2 was higher in involved nerves and muscles, and that DL-reconstruction slightly decreased DESS-T2. Additionally, DESS enabled analysis of the magic angle effect, demonstrating a positive correlation between nerve orientation and DESS-T2 values. |
| 16:01 |
0666.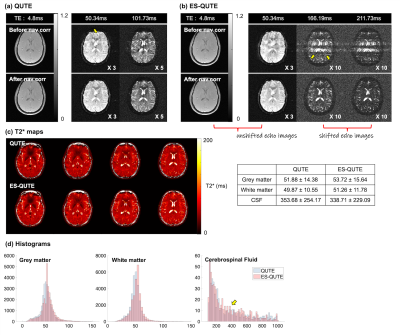 |
Development of a novel sequence for T2* quantification of
slow-relaxing water pools in the brain
Seonyeong Shin*1,2,
Ana-Maria Oros-Peusquens*1,
Seong Dae Yun1,
Ezequiel Farrher1,
and N. Jon Shah1,2,3,4,5
1Institute of Neuroscience and Medicine 4, INM-4, Forschungszentrum Jülich, Jülich, Germany, 2RWTH Aachen University, Aachen, Germany, 3Institute of Neuroscience and Medicine 11, INM-11, JARA, Forschungszentrum Jülich, Jülich, Germany, 4JARA - BRAIN - Translational Medicine, Aachen, Germany, 5Department of Neurology, RWTH Aachen University, Aachen, Germany Keywords: Quantitative Imaging, Relaxometry, Glympathics, CSF T2* relaxation in the brain covers a broad range of values, which can be grouped in three, roughly logarithmically spaced, intervals (short, intermediate, very long). Using a multi-echo GRE sequence to quantify T2* is time-efficient for brain parenchyma, but accurate quantification of slow-relaxing water pools, such as CSF, lengthens the acquisition time. In this work, we propose a novel sequence (ES-QUTE) that combines multi-echo acquisition with echo shifting techniques to effectively quantify the whole range of T2* relaxation times in the brain without increasing the scan time. In addition, ES-QUTE simultaneously characterises fast diffusion. |
| 16:09 |
0667.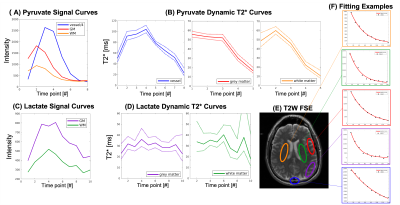 |
Dynamic T2* Relaxometry of Hyperpolarized [1-13C]pyruvate MRI in
the Human Brain and Kidneys
Xiaoxi Liu1,
Di Cui1,
Duan Xu1,
Robert Bok1,
Zhen J. Wang1,
Daniel B Vigneron1,2,
Peder E.Z. Larson1,2,
and Jeremy W. Gordon1 1Department of Radiology & Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2Graduate Program in Bioengineering, University of California, Berkeley and San Francisco, San Francisco, CA, United States Keywords: Quantitative Imaging, Hyperpolarized MR (Non-Gas) We present dynamic T2* measurements for HP [1-13C]pyruvate and metabolites in a healthy human brain volunteer and two RCC patients at 3T. The T2* of pyruvate was shown to vary during the acquisition, whereas the T2* of lactate and bicarbonate was constant through time and across organs. The T2* of lactate was constant at gray matter (30.1±5.9ms), white matter (33.8±7.6ms), healthy kidney (38.94±6.9ms) and tumor (33.27±6.4ms), and the T2* of bicarbonate over whole-brain (109.5±12.8ms) and kidney (64.6±15.8ms). These relaxometry measurements will be useful for future sequence optimization and can be included in kinetic modeling to harmonize data across different TEs. |
| 16:17 |
0668.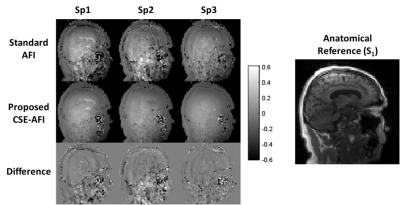 |
Accurate B1 Mapping with Actual Flip Angle Imaging (AFI) in the
Presence of Fat
Alexey Samsonov1,
Julia Velikina2,
and Vasily Yarnykh3 1Radiology, University of Wisconsin, Middleton, WI, United States, 2Radiology, University of Wisconsin, Madison, WI, United States, 3University of Washington, Seattle, WA, United States Keywords: Quantitative Imaging, Artifacts, B1 mapping; quantitative; parameter mapping Actual Flip angle Imaging (AFI) is an efficient B1 mapping method requiring the proper spoiling of transverse magnetization. Optimal spoiling can be achieved using large spoiling gradients enabling water diffusion-based signal decay. However, spoiling the non-aqueous signal like from fat is typically ignored in AFI optimizations. We demonstrate that infinitesimal diffusion in the fat signal makes fat spoiling in AFI unachievable in the reasonable scan time and that incomplete fat spoiling is a major source of previously unexplained AFI errors. We propose method to minimize them using a superposition model of the spoiling artifacts and chemical shift encoded fat/water separation. |
| 16:25 |
0669.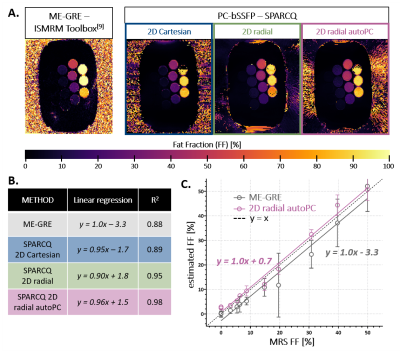 |
Golden-angle radial automated phase-cycled bSSFP for fat-water
decomposition with SPARCQ: validation in a custom phantom and in
vivo
Adèle L.C. Mackowiak1,2,3,
Jérôme Yerly1,4,
Katarzyna Pierzchala5,
Eva S. Peper2,3,
Giulia M.C. Rossi1,
and Jessica A.M. Bastiaansen2,3
1Department of Radiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 2Department of Diagnostic, Interventional and Pediatric Radiology (DIPR), University of Bern, Bern, Switzerland, 3Translational Imaging Center (TIC), Swiss Institute for Translational and Entrepreneurial Medicine, Bern, Switzerland, 4Center for Biomedical Imaging (CIBM), Lausanne, Switzerland, 5Laboratory of Functional and Metabolic Imaging, Swiss Federal Institute of Technology (EPFL), Lausanne, Switzerland Keywords: Quantitative Imaging, Fat, phase-cycled bSSFP, fat fraction mapping, radial The Signal Profiles Asymmetries for Robust multi-Compartment Quantification (SPARCQ) framework uses the off-resonance information encoded in phase-cycled bSSFP (PC-bSSFP) data to estimate fat fraction (FF). In order to strengthen previous validation work as well as open the range of applicability of the technique, in this work a 2D radial bSSFP sequence with integrated automated phase-cycling was designed and the accuracy of SPARCQ was tested in vitro on a larger FF range than previously reported. Comparisons to reference methods and sampling schemes indicate that the proposed automated 2D radial sampling scheme allows accurate FF mapping with SPARCQ while improving scan efficiency. |
| 16:33 |
0670.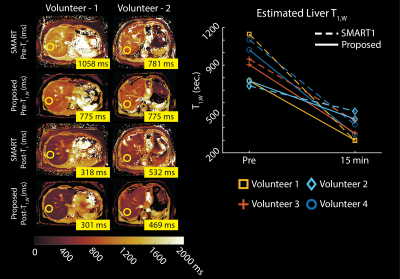 |
Free-Breathing, Confounder-Corrected, 3D T1 Mapping of the Liver
through Simultaneous Estimation of T1, PDFF, R2* and B1+
Yavuz Muslu1,2,
Ty A. Cashen3,
Sagar Mandava4,
Diego Hernando2,5,
and Scott B. Reeder1,2,5,6,7
1Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Global MR Applications and Workflow, GE Healthcare, Waukesha, WI, United States, 4Global MR Applications and Workflow, GE Healthcare, Atlanta, GA, United States, 5Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 6Department of Medicine, University of Wisconsin-Madison, Madison, WI, United States, 7Department of Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States Keywords: Quantitative Imaging, Liver, Multi-Contrast T1 relaxation is emerging as a biomarker for the diagnosis and staging of chronic liver disease. Modified Look-Locker Inversion Recovery (MOLLI) is widely used in clinical practice for abdominal T1 mapping; however, current methods are not corrected for fat and B1 inhomogeneities as confounding factors and fail to provide reliable T1 measurements. In this work, we propose a novel, free-breathing, confounder-corrected T1 mapping method over the entire liver, by combining inversion recovery and chemical shift encoding imaging for simultaneous estimation of T1, PDFF, R2* and B1+. |
| 16:41 |
0671.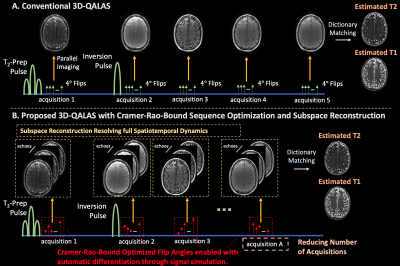 |
Improved T1 and T2 mapping in 3D-QALAS using temporal subspaces
and Cramer-Rao-bound flip angle optimization enabled by
auto-differentiation
Yamin Arefeen1,
Yohan Jun2,3,
Borjan Gagoski4,
Berkin Bilgic2,3,
and Elfar Adalsteinsson1,5,6
1Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 2Department of Radiology, Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 3Department of Radiology, Harvard Medical School, Boston, MA, United States, 4Department of Radiology, Boston Children's Hospital, Boston, MA, United States, 5Harvard-MIT Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States, 6Institute for Medical Engineering and Science, Massachusetts Institute of Technology, Cambridge, MA, United States Keywords: Quantitative Imaging, Pulse Sequence Design 3D-QALAS utilizes an interleaved Look-Locker acquisition with T2-preparation pulses for full brain quantification of T1 and T2. The sequence applies constant flip-angles and suffers from blurring due to k-space modulation from signal evolution during the lengthy echo-train. This abstract improves 3D-QALAS by (1) resolving the full temporal signal with subspace reconstructions to eliminate blurring, (2) optimizing acquisition flip angles with the Cramer-Rao-Bound using simulations compatible with auto-differentiation, and (3) decreasing the number of acquisitions within a repetition time, which could enable up to 40% reduced scan time. Simulation, phantom, and in-vivo results demonstrate the efficacy of the proposed sequence improvements. |
| 16:49 |
0672.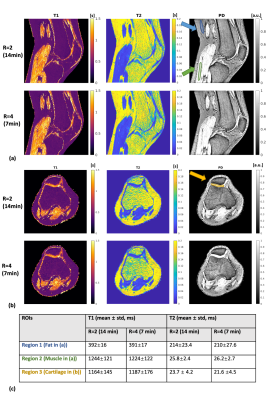 |
High-resolution three-dimensional MR-STAT for musculoskeletal
applications
Hongyan Liu1,
Oscar van der Heide1,
Edwin Versteeg1,
Miha Fuderer1,
Fei Xu1,
Martijn Froeling2,
Cornelis A.T. van den Berg1,
and Alessandro Sbrizzi1
1Computational Imaging Group, Department of Radiotherapy, University Medical Center Utrecht, Utrecht, Netherlands, 2Department of Radiology, Imaging Division, University Medical Center Utrecht, Utrecht, Netherlands Keywords: Quantitative Imaging, Quantitative Imaging MR-STAT is a framework for simultaneously acquiring multi-parametric quantitative maps from one single short scan. In this work, we design a new 3D MR-STAT sequence and the corresponding two-step reconstruction strategy based on previous work. The framework is improved by designing a faster acquisition framework, and more accurate signal modeling for reconstruction. The proposed sequence takes 7 minutes after retrospectively undersampling, and is validated in a phantom experiment. Furthermore, we apply this 3D MR-STAT sequence the first time for musculoskeletal applications. Knee and lower-leg experiments of healthy volunteers are shown here. |
| 16:57 |
0673.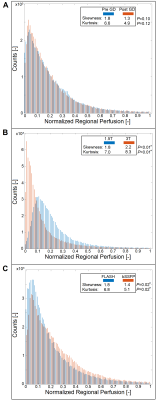 |
Influence of gadolinium, field-strength and sequence on
quantified perfusion values in phase-resolved functional lung
MRI
Julian Glandorf1,2,
Fynn Brunzema1,2,
Filip Klimes1,2,
Lea Behrendt1,2,
Andreas Voskrebenzev1,2,
Marcel Gutberlet1,2,
Robert Grimm3,
Frank Wacker1,2,
and Jens Vogel-Claussen1,2 1Institute for Diagnostic and Interventional Radiology, Hannover Medical School, Hannover, Germany, 2Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), Member of the German Centre for Lung Research (DZL), Hannover, Germany, 3MR Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany Keywords: Quantitative Imaging, Perfusion, Lung The aim of this study is the evaluation of the influences of previously applied gadolinium-based contrast media, differing field strength and differing imaging sequence on the lately proposed perfusion quantification for PREFUL MRI. The results indicate severe alterations and distortions of the quantified perfusion parameters after gadolinium administration and depending on the field strength and on the applied sequence type. The results show, that future multicenter studies need to strictly adhere to an identical imaging protocol using the same field strengths to generate comparable results across all sites. |
| 17:05 |
0674.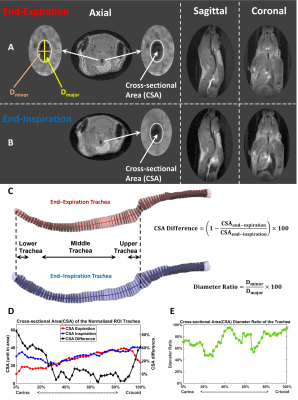 |
Quantifying Free-breathing Murine Tracheal Dynamics Using
Retrospectively-Gated Ultra-short Echo-time (UTE) in MRI
Qing Wang1,
Qiwei Xiao1,
Elizabeth M. Fugate2,
Matthew M. Willmering1,
Dianna M. Lindquist2,
Nana S. Higano1,2,3,
Alister J. Bates1,2,3,4,
and Zackary I. Cleveland1,2,3,4
1Pulmonary Medicine, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 2Radiology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 3Pediatrics, University of Cincinnati, Cincinnati, OH, United States, 4Biomedical Engineering, University of Cincinnati, Cincinnati, OH, United States Keywords: Quantitative Imaging, Preclinical, Ultra-short Echo-time (UTE), trachea The trachea expands and retracts almost uniformly while breathing, but these dynamics are altered by congenital malformation and injury to tracheal cartilage. Tracheal collapse—tracheomalacia—is a common comorbidity in of disorders, including bronchopulmonary dysplasia (BPD), idiopathic pulmonary fibrosis (IPF) and cystic fibrosis (CF) and can cause life-threatening airway obstruction. While tracheomalacia is clinically diagnosed via bronchoscopy, no tool exists to noninvasively assess tracheal dynamics in small animal models. Here we use retrospectively gated, 3D UTE to resolve changes in tracheal caliber during tidal breathing and show these dynamics change as a function of tracheal position and breathing rate. |
| 17:13 |
0675.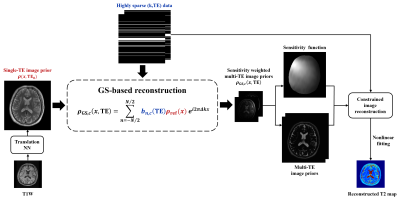 |
Accelerated T2 Mapping with GS-Assisted Deep Translation of T1W
Image Prior
Ruihao Liu1,2,
Yudu Li2,3,
Rong Guo2,4,
Yibo Zhao2,5,
Ziyu Meng1,
Huixiang Zhuang1,
Tianyao Wang6,
Yao Li1,
Yiping P. Du1,
and Zhi-Pei Liang2,5
1School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China, 2Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 3National Center for Supercomputing Applications, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 4Siemens Medical Solutions USA, Inc., Urbana, IL, United States, 5Department of Electrical and Computer Engineering, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 6Radiology Department, The Fifth People's Hospital of Shanghai, Shanghai, China Keywords: Quantitative Imaging, Machine Learning/Artificial Intelligence, T2 mapping Deep learning (DL)-based methods have shown great potential for accelerating T2W imaging by using image prior generated from a companion T1W image. However, quantitative T2 mapping requires multiple T2W images acquired with multi-TE, creating practical problem for the use of DL for accelerated T2 mapping due to insufficient multi-TE training data. This work addresses this problem by using a generalized series model to map a T2W DL prior for one TE to multiple TEs, enabling effective use of T1W image prior for high-quality T2 mapping from sparsely sampled data. The method has been validated using experimental data, producing impressive results. |
17:21 |
0676.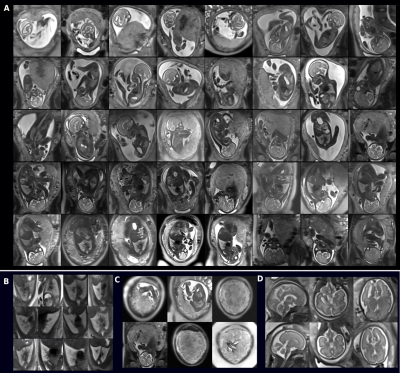 |
Widening access and increasing value to fetal MRI with a
clinical 20min low-field 0.55T fetal exam in 40 participants
Jordina Aviles Verdera1,2,
Lisa Story1,3,
Megan Hall1,3,
Tom Finck1,4,
Alexia Egloff1,5,
Shaihan Malik1,2,
Mary A Rutherford1,2,
Joseph V Hajnal 1,2,
Raphael Tomi-Tricot1,2,6,
and Jana Hutter1,2
1Center for the Developing Brain, School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom, 2Biomedical Engineering Department, School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom, 3Women's Health, Guy's and St. Thomas' NHS Foundation Trust, London, United Kingdom, 4Department for Diagnostic and Interventional Neuroradiology, Klinikum rechts der Isar Muenchen, Munich, Germany, 5Radiology, Guy's and St. Thomas' NHS Foundation Trust, London, United Kingdom, 6MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom Keywords: Quantitative Imaging, Fetus Comprehensive anatomical and functional fetal examination at low field (0.55T) MRI, obtained using a 20min protocol in 40 pregnant women demonstrates high quality data suitable for quantitative analysis both for anatomical radiological values and for functional T2* and diffusion values, all in-line with previously reported higher field values. It opens novel avenues and widens access to fetal MRI to new groups such as the fast growing fraction of obese and overweight pregnant women, in whom comprehensive assessment by screening ultrasound may be difficult. |
| 17:29 |
0677.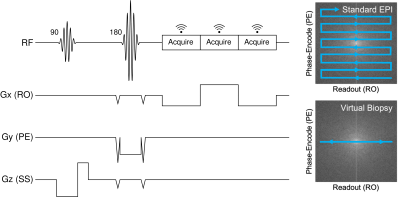 |
Evaluating Echo Planar Spectroscopic Imaging with a Columnar
Excitation for "Virtual Biopsies"
Michael S Yao1,2,
Andrew Van3,4,
James Gee2,
Murray Grossman5,
David J Irwin5,6,
and M Dylan Tisdall2
1Department of Bioengineering, University of Pennsylvania, Philadelphia, PA, United States, 2Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 3Department of Neurology, Washington University School of Medicine, St. Louis, MO, United States, 4Department of Biomedical Engineering, Washington University School of Medicine, St. Louis, MO, United States, 5Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 6Pathology and Laboratory Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States Keywords: Quantitative Imaging, Quantitative Imaging The acquisition of high-resolution quantitative measurements is of particular interest in studying the laminar structure and layer-specific pathology in the cerebral cortex. In this work, we propose a method to address this need by acquiring 1-D echo planar spectroscopic imaging (EPSI) as a "virtual biopsy" with 200 µm resolution along the readout direction. Our sequence yields expected spectra for common compounds and produces high-quality quantitative T2* and off-resonance measurements in ex vivo brain tissue. Our goal is to use this quantitative virtual biopsy to discriminate laminar variations in cortical iron deposition in diseases such as frontotemporal lobar degeneration (FTLD). |
17:37 |
0678.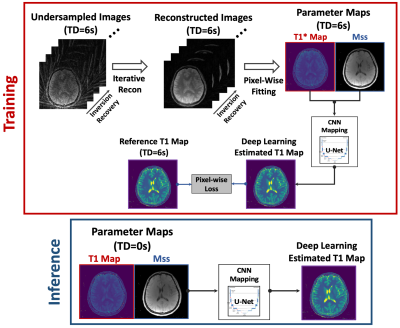 |
Rapid 3D T1 Mapping Using Deep Learning-Assisted Look-Locker
Inversion Recovery MRI
Haoyang Pei1,2,
Ding Xia1,
Xiang Xu1,
Yang Yang1,3,
Yao Wang2,
Fang Liu4,
and Li Feng1
1Biomedical Engineering and Imaging Institute and Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Department of Electrical and Computer Engineering, NYU Tandon School of Engineering, New York, NY, United States, 3Department of Radiology and Biomedical Imaging, UCSF, San Francisco, CA, United States, 4Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States Keywords: Quantitative Imaging, Quantitative Imaging Look-Locker inversion recovery (LLIR) imaging is an easy, accurate and reliable MRI method for T1 mapping. For 3D acquisition, LLIR imaging is usually performed with multiple repetitions, and additional idle time is placed between consecutive receptions. This idle time allows for signal recovery to improve SNR and ensures robustness to B1 inhomogeneity, but it also prolongs scan time. Simply eliminating the idle time reduces the accuracy of T1 quantification. In this work, a novel deep-learning approach was proposed to address this challenge, so that accurate 3D T1 maps can be generated from continuous 3D LLIR imaging without idle time. |
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.