Power Pitch
Pitch: Cardiovascular Solid Modeling: Advanced Tissue Characterization
ISMRM & ISMRT Annual Meeting & Exhibition • 03-08 June 2023 • Toronto, ON, Canada

| 16:00 |
0329.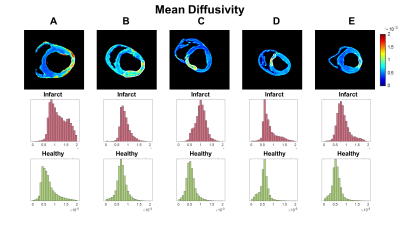 |
Myocardial Infarction: An Investigation of Mesostructure
using Diffusion Tensor Imaging and Voxel-Based Analysis
Alexander James Wilson1,2,3,
Xiaojian Kang2,
Tyler Cork1,
Luigi Perotti4,
and Daniel Ennis1,2,3
1Radiological Sciences Laboratory, Stanford University, Stanford, CA, United States, 2Division of Radiology, Veterans Administration Health Care System, Palo Alto, CA, United States, 3Cardiovascular Institute, Stanford University, Stanford, CA, United States, 4Department of Mechanical and Aerospace Engineering, University of Central Florida, Orlando, FL, United States Keywords: Myocardium, Diffusion Tensor Imaging Ex vivo DTI was performed in hearts suffering from myocardial infarction. Data was reconstructed using both tensor-based and voxel-based analysis. The fixel number (FN) consistently showed that there was a single fiber population (FN=1) in both infarcted and healthy myocardium. The complexity (CX) indicates the spread and magnitude of diffusion along different directions, but was not significantly different between infarct and healthy regions. The apparent fiber density (AFD) reveals the density of each fiber population within a voxel, and curiously increased in the infarct region, which may indicate the compaction of fiber populations running through the infarct. |
| 16:00 |
0330.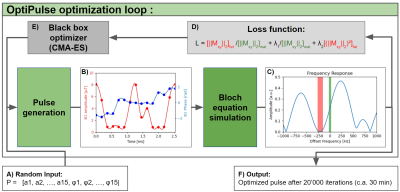 |
Efficient fat suppression in free-running whole-heart CMR
using OptiPulse
Xavier Sieber1,
Ludovica Romanin1,2,
Chris W. Roy1,
Jessica AM Bastiaansen3,4,
Jérôme Yerly1,
Jonas Richiardi1,
Matthias Stuber1,5,
and Ruud B. van Heeswijk1
1Department of Diagnostic and Interventional Radiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 2Advanced Clinical Imaging Technology, Siemens Healthineers International AG, Lausanne, Switzerland, 3Department of Diagnostic, Interventional and Pediatric Radiology (DIPR), Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland, 4Translation Imaging Center (TIC), Swiss Institute for Translational and Entrepreneurial Medicine, Bern, Switzerland, 5Division of Cardiology, CIBM Center for Biomedical Imaging, Lausanne, Switzerland Keywords: Vessels, RF Pulse Design & Fields The incomplete or time-inefficient suppression of fat signals is an unsolved issue in 3D cardiovascular MR imaging (CMR). We present a flexible framework named OptiPulse to design spectrally-selective radiofrequency (RF) pulses using numerical optimization of Bloch equation simulations. The RF pulse performance was assessed using a composite loss function for both fat suppression and power requirement. OptiPulse was used to design a B-splines-interpolated pulse for use in a free-running 3D whole-heart pulse sequence. Its fat suppression performance was ascertained both in vitro and in vivo, where it resulted in more homogeneous fat suppression when compared to other WE pulses (P<0.01). |
| 16:00 |
0331.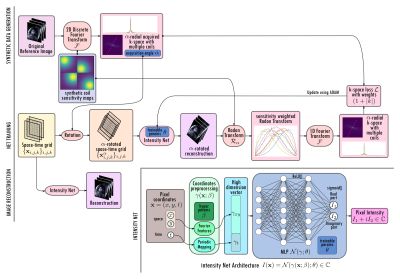 |
Highly accelerated Cardiac CINE MRI using Neural Fields
Tabita Catalán1,
Matías Courdurier1,2,
Axel Osses1,3,
René Botnar1,4,5,
Francisco Sahli Costabal1,4,
and Claudia Prieto1,4
1Millennium Nucleus For Applied Control And Inverse Problems, Santiago, Chile, 2Department of Mathematics, Pontificia Universidad Católica de Chile, Santiago, Chile, 3Department of Mathematical Engineering, Universidad de Chile, Santiago, Chile, 4School of Engineering, Pontificia Universidad Católica de Chile, Santiago, Chile, 5Institute for Biological and Medical Engineering, Pontificia Universidad Católica de Chile, Santiago, Chile Keywords: Image Reconstruction, Heart Neural fields cardiac MRI (NF-cMRI), a method for highly accelerated CINE reconstruction using deep learning, is proposed. NF-cMRI relies on an intensity network, based on neural fields with Fourier features to encode a continuous reconstruction. The network is trained with one undersampled radial k-space data set without the need of a fully-sampled reference image. Good image quality of the heart is achieved with 8 radial spokes/cardiac frame. Results are compared against GRASP. Future work will focus on reducing reconstruction time and evaluating the proposed approach in prospectively undersampled k-space data. |
| 16:00 |
0332.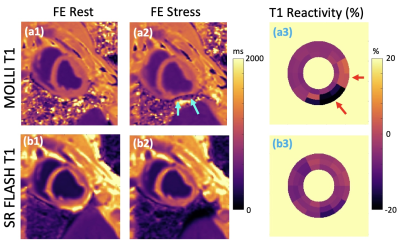 |
Free-breathing mapping of stress/rest myocardial T1
reactivity with Ferumoxytol-enhanced imaging using a widely
available sequence
Hazar Benan Unal1,
Shahriar Zeynali1,
Subha Raman2,
Balaji Tamarappoo2,
Rohan Dharmakumar2,
and Behzad Sharif1,2
1Laboratory for Translational Imaging of Microcirculation, Indiana University School of Medicine, Indianapolis, IN, United States, 2Krannert Cardiovascular Research Center, Indiana University School of Medicine, Indianapolis, IN, United States Keywords: Myocardium, Ischemia, dobutamine Myocardial T1 reactivity, defined as the relative T1 change from rest to stress, has been proposed as a marker for detection of ischemic heart disease. Commonly used MOLLI T1 mapping is sensitive to B0 field inhomogeneities and can have susceptibility/banding artifacts because of bSSFP readouts, especially at 3T. In this study, we investigated the feasibility of free-breathing SR-FLASH T1 mapping for ferumoxytol-enhanced (FE) dobutamine-stress T1 reactivity studies at 3T in preclinical settings. We showed the feasibility of using a widely available perfusion sequence for T1 reactivity studies under Ferumoxytol-enhancement. |
| 16:00 |
0333.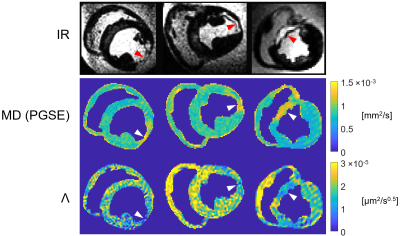 |
Diffusion dispersion mapping of ischemic lesions in the ex
vivo porcine heart
Eric S. Michael1,
Claire A. Dick1,
Franciszek Hennel1,
Christian T. Stoeck1,2,
and Klaas P. Pruessmann1
1Institute for Biomedical Engineering, ETH Zurich and University of Zurich, Zurich, Switzerland, 2Center for Surgical Research, University Hospital Zurich, University of Zurich, Zurich, Switzerland Keywords: Heart, Ischemia Diffusion dispersion mapping is a recently proposed method for probing tissue microstructure and quantifying microstructural disorder based on differences in the impact of diffusion restrictions over different length scales. In this work, this method was employed in the ex vivo heart to investigate the utility of diffusion dispersion in identifying and characterizing ischemic tissue. Our results show that ischemic cardiac lesions exhibit reduced diffusion dispersion rates, in agreement with known microstructural changes, and can be differentiated with respect to surrounding tissue. These findings demonstrate that microstructure-sensitive contrast offers a novel avenue for probing and characterizing cardiac pathologies. |
| 16:00 |
0334.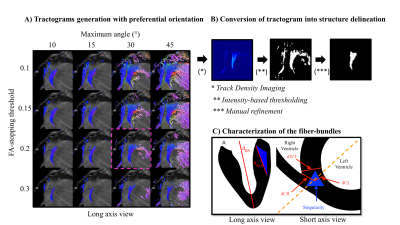 |
Cardiac structure discontinuities revealed by ex-vivo
microstructural characterization on the Basal Inferoseptal
Left Ventricle region.
Pierre CABANIS1,2,
Julie MAGAT1,2,
Jairo RODRIGUEZ-PADILLA3,
Girish RAMLUGUN2,
Maxime YON2,
Yann BIHANPOUDEC4,
Nestor PALLARES-LUPON2,
Fanny VAILLANT2,
Philippe PASDOIS2,
Pierre JAIS2,5,
Pierre DOS-SANTOS2,5,
Marion CONSTANTIN2,
David BENOIST2,
Line POURTAU2,
Virginie DUBES2,
Julien ROGIER2,5,
Louis LABROUSSE2,5,
Michel HAISSAGUERRE2,5,
Olivier BERNUS2,
Bruno QUESSON1,2,
Richard WALTON2,
Josselin DUCHATEAU2,5,
Edward VIGMOND2,
and Valéry OZENNE1,2
1Univ. Bordeaux, CNRS, CRMSB, UMR 5536, Bordeaux, France, 2Liryc, Electrophysiology and Heart Modeling Institute, Fondation Bordeaux Université, Pessac-Bordeaux, France, 3Inria Epione Team, Université Côte d'Azur, Biot, France, 4Centre de Neuroscience Cognitive, CNRS, Université Claude Bernard Lyon I, Villeurbanne, France, 5Cardiology Departement, Bordeaux University Hospital (CHU), Pessac, France Keywords: Myocardium, Microstructure, Fiber The knowledge of the cardiac microstructure and the 3D myofiber architecture grow years after years with the multiplication and the upgrade of imaging technologies. However, the course of events of pathophysiological processes like cardiac remodeling, and the link with clinical phenotypes are not yet clearly understood. Some concerns have been raised regarding the interpretation of the late gadolinium enhancement (LGE) at the right ventricle attachment or insertion point (RVIP) however 3D microstructure organization of the RVIP has not been extensively described in the literature. |
| 16:00 |
0335.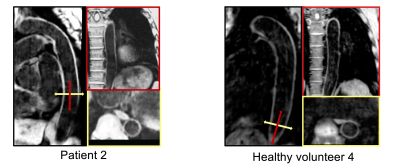 |
3-Dimensional DANTE-prepared sequence with non-rigid
motion-correction for high-resolution dark-blood aortic
imaging
Anastasia Fotaki1,
Camila Munoz1,
Alina Hua1,
Karl P Kunze1,2,
Radhouene Neji1,2,
Tevfik F Ismail1,
Rene M Botnar1,3,4,5,
and Claudia Prieto1,3,4,5
1Biomedical Engineering, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Camberley, United Kingdom, 3Institute for Biological and Medical Engineering, Pontificia Universidad Católica de Chile, Santiago, Chile, 4Millennium Institute for Intelligent Healthcare Engineering, Santiago, Chile, 5School of Engineering, Pontificia Universidad Católica de Chile, Santiago, Chile Keywords: Vessels, New Signal Preparation Schemes, Pulse Sequence Design Dark-blood imaging is an important tool for vascular imaging in cardiovascular disease. A novel free-breathing water/fat DANTE-prepared sequence is introduced for 3D dark-blood imaging of the thoracic and abdominal aorta. The framework integrates image navigation to enable translational and non-rigid motion correction resulting in a predictable scan time along with dual-echo Dixon gradient-echo readout for robust fat suppression. Results from healthy subjects and patients demonstrate the feasibility of the sequence for high-resolution, aortic imaging in ~7-minute acquisition time. |
| 16:00 |
0336.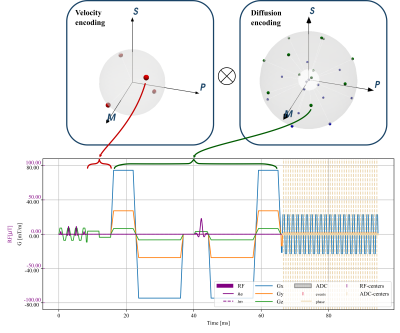 |
Joint cardiac tissue velocity and diffusion tensor mapping
Jonathan Weine1,
Stefano Buoso1,
Charles McGrath1,
and Sebastian Kozerke1
1University and ETH Zurich, Zurich, Switzerland Keywords: Heart, Diffusion Tensor Imaging Myocardial strains and microstructure are considered important indicators of muscle contractility and function, which can be investigated with cardiac diffusion tensor imaging (cDTI) and tissue-velocity mapping. In this work we perform simulations incorporating contractile motion and model based diffusion contrast to investigate jointly encoding the information by modifying existing cDTI protocols. Key characteristics of the results agree with practical findings and suggest sufficient estimation accuracy for velocities and diffusion tensors. |
| 16:00 |
0337.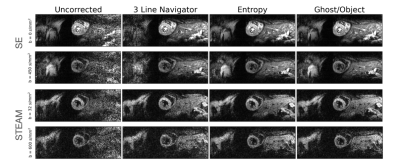 |
Referenceless Nyquist Ghost Correction Outperforms Standard
Navigator Based Method for DT-CMR
Zimu Huo1,2,
Ke Wen1,
Yaqing Luo1,
Pedro F Ferreira1,
Radhouene Neji3,4,
Dudley Pennell1,
Andrew D Scott1,
and Sonia Nielles-Vallespin1
1CMR Unit, Royal Brompton Hospital and NHLI, Imperial college London, London, United Kingdom, 2Department of Bioengineering, Imperial College London, London, United Kingdom, 3School of Biomedical Engineering and Imaging Sciences, King’s College London, London, United Kingdom, 4MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom Keywords: Myocardium, Diffusion Tensor Imaging Nyquist ghosting is a common artefact in echo planar imaging (EPI), typically corrected using separately acquired reference data. Here we demonstrate that reference free Nyquist ghost correction algorithms based on entropy and Ghost/Object ratio minimization can outperform navigator based methods and improve imaging efficiency for in vivo diffusion tensor cardiovascular magnetic resonance. |
| 16:00 |
0338.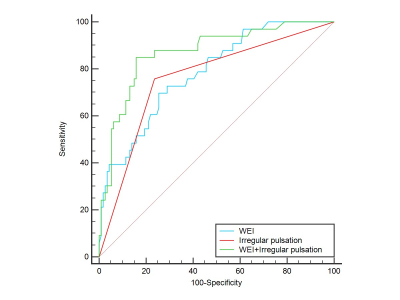 |
Combination of irregular pulsation and aneurysm wall
enhancement improved the diagnostic efficiency of
symptomatic intracranial aneurysm
Xiao Li1,
Jianjian Zhang1,
Huilin Zhao1,
and Chengcheng Zhu2
1Ren ji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, China, 2University of Washington, Seattle, WA, United States Keywords: Vessels, Vessels Both aneurysm wall enhancement (AWE) and irregular pulsation have been suggested as potential candidates for intracranial aneurysms (IAs) instability. However, no studies have compared irregular pulsation and AWE for evaluation symptoms in unruptured IAs. By using vessel wall MRI and four-dimensional computed tomography angiography, we found combination of aneurysm wall enhancement and irregular pulsation improve the diagnostic efficiency of symptomatic intracranial aneurysm compared with irregular pulsation or AWE alone. Further longitudinal studies are needed to validate the role of the two imaging markers in predicting aneurysm growth and rupture. |
| 16:00 |
0339.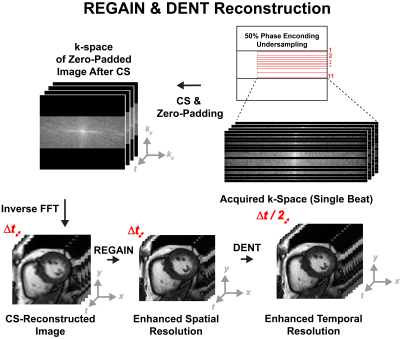 |
Highly-Accelerated High-Frame-Rate Cine for Exercise Cardiac
MRI
Manuel Morales1,
Manuel A Morales1,
Siyeop Yoon1,
Jennifer Rodriguez1,
Warren J Manning1,
and Reza Nezafat1
1BIDMC, Boston, MA, United States Keywords: Heart, Machine Learning/Artificial Intelligence Combined cardiac MRI with exercise (Ex-CMR) is a stress test with promising applications. However, standard ECG-segmented cine imaging during exercise is challenging. Free-breathing ECG-free real time cine can be achieved with compressed sensing. Yet tradeoff remains between temporal and spatial temporal resolution. Thus, we sought to develop a highly accelerated high-frame-rate cine for Ex-CMR by accelerating spatial resolution using Resolution Enhancement Generative Adversarial Inline Network (REGAIN), followed by synthesizing new frames using Deformation ENcoding Transformer (DENT). REGAIN enabled 14-fold scan acceleration, DENT enabled 2-fold improvement in temporal resolution. We achieved spatiotemporal resolution of 1.9 × 1.9 mm2 and 16 ms. |
| 16:00 |
0340.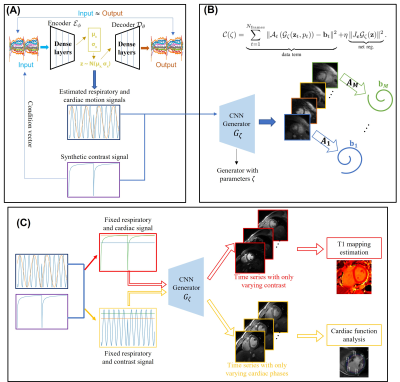 |
Joint cardiac $$$T_1$$$ mapping and cardiac cine using a
deep manifold framework
Qing Zou1,
Sarv Priya2,
Prashant Nagpal3,
and Mathews Jacob2
1University of Texas Southwestern Medical Center, Dallas, TX, United States, 2University of Iowa, Iowa City, IA, United States, 3University of Wisconsin–Madison, Madison, WI, United States Keywords: Heart, Machine Learning/Artificial Intelligence, Reconstruction The main focus of this work is to introduce a deep generative model for simultaneous free-breathing cardiac $$$T_1$$$ mapping and CINE MRI. The data is acquired by a gradient echo inversion recovery sequence with intermittent delays for magnetization recovery. The joint reconstruction of the image time-series is performed using a patient-specific deep manifold reconstruction algorithm which learns a CNN generative model and its latent vectors from the measured k-t space data in an unsupervised fashion. Following learning, the model can be used to generate synthetic images at specific motion and contrast states. |
| 16:00 |
0341.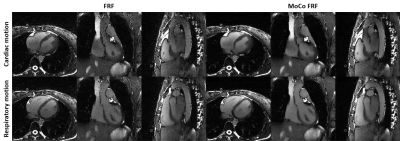 |
High on Sparsity: Inter-Bin Compensation of Cardiac Motion
for Improved Assessment of Left Ventricular Function Using
5D whole-heart MRI
Jérôme Yerly 1,2,
Christopher W Roy1,
Milan Prsa3,
Bastien Milani1,
and Matthias Stuber1,2
1Department of Diagnostic and Interventional Radiology, Lausanne University Hospital, Lausanne, Switzerland, 2Center for Biomedical Imaging (CIBM), Lausanne, Switzerland, 3Woman-Mother-Child Department, Lausanne University Hospital, Lausanne, Switzerland Keywords: Heart, Image Reconstruction The reconstruction of 5D cardiac and respiratory motion-resolved images with the free-running framework (FRF) requires careful optimization of the regularization weights to avoid compression of physiological motion, which in turn may lead to underestimated left ventricular ejection fraction (LVEF). This study presents a novel cardiac and respiratory motion-resolved reconstruction with inter-bin compensation of cardiac motion. We demonstrate that the proposed framework significantly improves image quality while preserving accurate LVEF when compared to the original 5D framework. This work highlights important considerations when reconstructing cardiac resolved images without compensation and provides a robust approach for the assessment of LVEF. |
| 16:00 |
0342.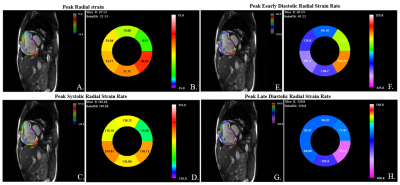 |
Characterize right ventricle deformation pattern in
pulmonary arterial hypertension using layer-specific strain
cardiovascular MRI
Wen Li1,
Xianchang Zhang2,
Jing An3,
Jens Wetzl4,
Qing Gu5,
and Jianguo He6
1State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China, 2MR Collaboration, Siemens Healthineers Ltd, Beijing, China, 3Siemens Shenzhen Magnetic Resonance Ltd, Shenzhen, China, 4Magnetic Resonance, Siemens Healthcare, Erlangen, Germany, 5Emergency Center, State Key Laboratory of Cardiovascular Disease, Key Laboratory of Pulmonary Vascular Medicine, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China, 6Center of Pulmonary Vascular Disease, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China Keywords: Myocardium, Rare disease, Strain, right ventricle This is the first study to describe the distribution pattern of right ventricular layer-specific strain/strain rate in pulmonary arterial hypertension patients by processing cine cardiovascular MR images using a deformation registration algorithm based strain analysis software. |
| 16:00 |
0343.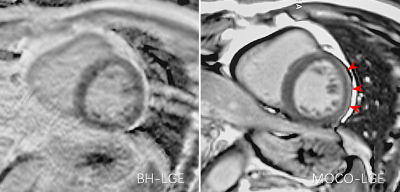 |
Diagnostic pearls and potential pitfalls of free-breathing
motion-corrected LGE: a prospective head-to-head comparative
study
Hui Zhou1,
Jing Luo1,
Huiting Zhang2,
and Xiaoming Bi3
1Department of Radiology, Xiangya Hospital Central South University, Changsha, China, 2Scientific Marketing, Siemens Healthineers Ltd., Wuhan, China, 3MR Collabration, Siemens Healthineers, Los Angeles, CA, United States Keywords: Cardiomyopathy, Myocardium, Late gadolinium enhancement This study investigated the advantages and potential disadvantages of the MOCO-LGE sequence. The results showed that MOCO-LGE can effectively improve the image quality compared to BH-LGE. Meanwhile, the MOCO-LGE images could overestimate epicardial hyperenhancement and pericardial effusion, which might lead to overdiagnosis of myocarditis in clinical work. The study indicates that further refinement of this useful sequence may be needed. |
| 16:00 |
0344.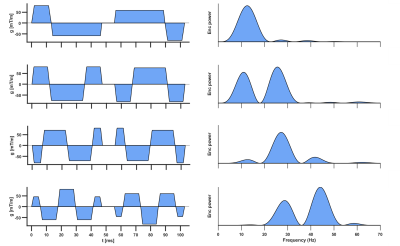 |
Time-dependent Diffusion in the Human Heart In Vivo
Irvin Teh1,
Sam Coveney1,
Richard J. Foster1,
Filip Szczepankiewicz2,
Samo Lasič3,4,
Henrik Lundell3,
David Shelley5,
Lars Mueller1,
Maryam Afzali1,6,
Noor Sharrack1,
Nadira Y. Yuldasheva1,
Sven Plein1,
Erica Dall'Armellina1,
and Jürgen E. Schneider1
1Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, United Kingdom, 2Medical Radiation Physics, Clinical Sciences Lund, Lund University, Lund, Sweden, 3Danish Research Centre for Magnetic Resonance, Centre for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital Amager and Hvidovre, Copenhagen, Denmark, 4Random Walk Imaging, Lund, Sweden, 5Leeds Teaching Hospitals Trust, Leeds, United Kingdom, 6Cardiff University Brain Research Imaging Centre (CUBRIC), School of Psychology, Cardiff University, Cardiff, United Kingdom Keywords: Myocardium, Diffusion Tensor Imaging, Time dependence, microstructure, motion compensation Conventional spin-echo based cardiac diffusion tensor imaging (DTI) has a relatively limited range of encoding frequencies, and hence limited sensitivity to diffusion at different length scales. Here, we explored the feasibility of applying a broader range of frequencies to evaluate the effects of time-dependent diffusion. We employed diffusion encoding waveforms with up to 4th order motion-compensation in a cohort of healthy volunteers, and report trends of decreasing MD and FA with increasing encoding frequencies. The availability of higher frequencies enhances the sensitivity of DTI to shorter length scales, and may be more greatly weighted towards diffusion properties of sub-cellular structures. |
| 16:00 |
0345. |
Simplified Protocol with mSAVA for 3D Imaging of T1, T2,
Extracellular Volume, Bright-Blood and Dark-Blood Late
Gadolinium Enhancement
Dongyue Si1,
Lan Cheng2,3,
Xiangchuang Kong2,3,
Rui Guo4,
Daniel A. Herzka5,
and Haiyan Ding1
1Center for Biomedical Imaging Research, Department of Biomedical Engineering, Tsinghua University, Beijing, China, 2Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, 3Hubei Province Key Laboratory of Molecular Imaging, Wuhan, China, 4School of Medical Technology, Beijing Institute of Technology, Beijing, China, 5Department of Radiology, Case Western Reserve University, Cleveland, OH, United States Keywords: Cardiomyopathy, Tissue Characterization Multi-parametric SAturation recovery and Variable flip Angle (mSAVA) acquires four 3D volumes with different T1 and T2 weightings during free-breathing to simultaneously generate whole heart T1 and T2 parametric maps. We proposed a fast and simple CMR protocol using pre- and post-contrast mSAVA acquisitions to additionally generate ECV maps, and bright-blood and dark-blood LGE images. mSAVA could comprehensively assess the myocardium over the whole heart within ~6+6 min in a cohort of twenty patients. |
| 16:00 |
0346.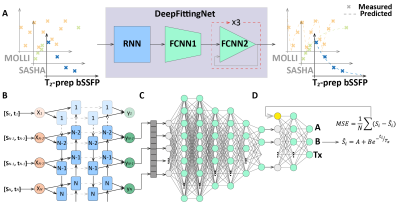 |
DeepFittingNet: a deep neural network-based approach for
simplifying cardiac T1 and T2 estimation with improved
robustness
Rui Guo1,
Dongyue Si2,
Yingwei Fan1,
Haina Zhang3,
Haiying Ding2,
and Xiaoying Tang4
1School of Medical Technology, Beijing Institute of Technology, Beijing, China, 2Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 3Center for Community Health Service, Peking University Health Science Center, Beijing, China, 4School of Life Science, Beijing Institute of Technology, Beijing, China Keywords: Myocardium, Machine Learning/Artificial Intelligence, Cardiac T1 and T2 mapping, myocardium tissue characterization The most used curve-fitting method for map reconstruction of the cardiovascular magnetic resonance mapping is sensitive to the initial conditions, time-consuming, and prone to fitting error. In this study, we sought to develop a deep-learning approach (DeepFittingNet) to perform T1 and T2 calculations for the most clinically used cardiac parametric mappings, to simplify the clinical workflow of cardiac T1/T2 measurements and improve the robustness. In testing, DeepFittingNet could perform T1/T2 estimation tasks for MOLLI, SASHA, and T2-prep bSSFP. Compared to the curve-fitting algorithm, DeepFittingNet could improve the robustness for inversion-recovery T1 estimation and have comparable accuracy and precision. |
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.