Power Pitch
Pitch: Ask Not What You Can Do for Your AI; Ask What Your AI Can Do for You: Razor's Edge in Neurovascular & Cardiovascular MRI
ISMRM & ISMRT Annual Meeting & Exhibition • 03-08 June 2023 • Toronto, ON, Canada

15:45 |
0694.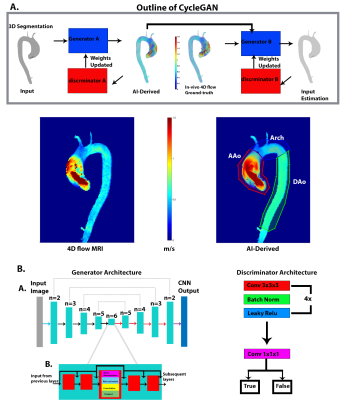 |
Highly Resilient 3D Aortic Hemodynamics derived directly
from Aortic Geometry using AI
Haben Berhane1,
Anthony Maroun1,
Mahmoud Ebrahimkhani1,
Ulas Bagci1,
Bradley Allen1,
and Michael Markl1
1Radiology, Northwestern University, Chicago, IL, United States Keywords: Flow, Velocity & Flow, CFD Aortic hemodynamic quantifications are vital for patient management. While 4D Flow MRI provides comprehensive aortic hemodynamics, it is hampered by long-acquisition times and cumbersome pre-processing. In this study, we developed an AI for the prediction of systolic 3D blood flow velocity vector fields with 3D aortic geometry as the only input. We performed testing on 248 BAV and 104 TAV datasets, using the systolic velocity vector fields from the 4D flow MRI as the ground-truth. Generally, we saw very strong agreement between the AI and the 4D flow and resilience to geometric changes (volume, dimension) in the input segmentation. |
| 15:45 |
0695.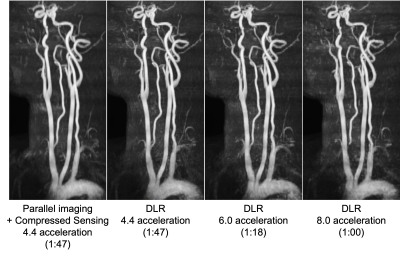 |
Highly accelerated FLEXA 3DTOF MR Angiography with iterative
deep learning reconstruction
Naoyuki Takei1,
Rafi Brada2,
Sangtae Ahn3,
Graeme McKinnon4,
Xucheng Zhu5,
Atsushi Nozaki1,
Shigeo Okuda6,
Masahiro Jinzaki6,
and Tetsuya Wakayama1
1GE Healthcare, Tokyo, Japan, 2GE Research, Herzliya, Israel, 3GE Research, Niskayuna, NY, United States, 4GE Healthcare, Waukesha, WI, United States, 5GE Healthcare, Menlo Park, CA, United States, 6Keio University School of Medicine, Tokyo, Japan Keywords: Vessels, Machine Learning/Artificial Intelligence Rapid non-contrast MRA of supra-aortic arteries is necessary to select proper patient for endovascular therapy (EVT) as EVT has become the predominant therapy of acute ischemic stroke. However, conventional 3DTOF has long scan time of 6-7 minutes to cover the entire carotid artery. A highly accelerated MRA with iterative deep learning reconstruction was developed to provide the wide scan coverage and less motion sensitivity within 1 minute. We evaluated the technique with prospectively and retrospectively undersampled data demonstrating high-quality vessel visualization and improved acquisition efficiency at 8x acceleration. |
| 15:45 |
0696.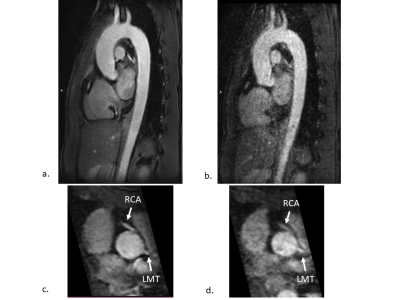 |
One-minute 3D ky-kz Centric FFE Thoracic Aorta Imaging with
Real-Time Motion-Correction and High-Resolution Deep
Learning Reconstruction
Hideki Ota1,
Satoshi Higuchi1,
Yoshiaki Morita2,
Takashi Nishina3,
Sho Tanaka3,
Yuichi Yamashita3,
Yoshimori Kassai3,
Tasuo Nagasaka1,
Mitsue Miyazaki4,
and Kei Takase1
1Tohoku University Hospital, Sendai, Japan, 2National Cardiovascular Research Center, Osaka, Japan, 3Canon Medical Systems, Otawara, Japan, 4University of California, San Diego, San Diego, CA, United States Keywords: Vessels, Vessels, workflow, deep learning reconstruction Efficiency of 3D ky-kz centric fast field echo (FFE) acquisition was acquired using 100% efficiency of real-time motion correction in the sagittal oblique acquisition and compared with conventional 3D FFE acquisition. The thoracic aorta was acquired within 1 minute using centric ky-kz FFE and reconstructed with high-resolution deep learning reconstruction (HR-DLR), providing good image quality. Regular FFE with conventional reconstruction provides fair image quality with prominent noise and artifacts. |
| 15:45 |
0697.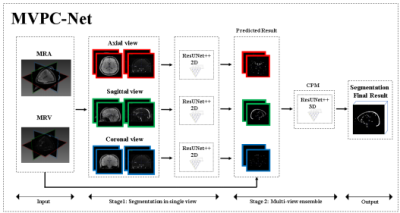 |
Fully Automated Segmentation of Brain and Scalp Blood
Vessels on Multi-Parametric Magnetic Resonance Imaging Using
Multi-view Cascaded Network
Yang Yang1,
songxiong wu2,
Bingsheng Huang3,
Ping Zeng2,
Mingyu Wang4,
and Zilong Huang4
1Department of Radiology, Suining Central Hospital, Suining, China, 2Radiology Department, Shenzhen University General Hospital and Shenzhen University Clinical Medical Academy, shenzhen, China, 3Medical AI Lab, School of Biomedical Engineering, Health Science Center, Shenzhen University, Shenzhen, China, 4Medical AI Lab, School of Biomedical Engineering, Health Science Center, Shenzhen University, shenzhen, China Keywords: Vessels, Blood, brain blood vessel segmentation,Multi-Parametric,Multi-view Accurate segmentation of blood vessels allow neurosurgical navigation and can help neurosurgeons accurate surgical and treatment plans. However, traditional blood vessel segmentation methods based on thresholds have limited performance. To solve problem, we proposed a cascaded DL network (MVPC-Net) that combines three refinements: multi-view learning, multi-parameter input, and a multi-view ensemble module-based strategy. The results of ablation experiments showed that, by adding all the refinements proposed, the performance of the baseline model improved from Dice similarity coefficient 0.865 to 0.922. Thus, our method can provide better segmentation of the brain, and scalp blood vessels and has potential for clinical application. |
| 15:45 |
0698.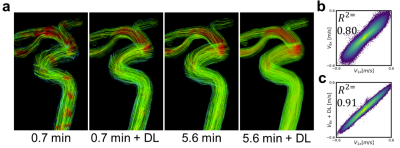 |
Denoising 4D-Flow using Self-Supervised Deep Learning and
its effect on test-rest reproducibility
Brock W Jolicoeur1,
Leonardo A Rivera-Rivera1,
Grant S Roberts1,
Laura Eisenmenger2,
and Kevin M Johnson1
1Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Radiology, University of Wisconsin-Madison, Madison, WI, United States Keywords: Flow, Blood vessels, Machine Learning/Artificial Intelligence Through a self-supervised deep learning denoising algorithm, more precise cerebrovascular measurements of flow, maximum fluid velocity, and vessel cross-sectional area were obtained from undersampled neurovascular 4D-Flow MRI data. This algorithm was applied to back-to-back scans to illustrate its effectiveness in reducing measurement variance. |
| 15:45 |
0699.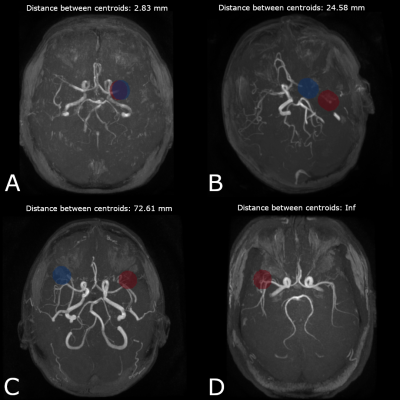 |
Large vessel occlusion detection on TOF images using deep
learning
Zakarya BENTATOU1,
Timothé BOUTELIER1,
Anais BERNARD1,
and Henitsoa RASOANANDRIANINA1
1OLEA MEDICAL, La Ciotat, France Keywords: Stroke, Machine Learning/Artificial Intelligence, Large vessel occlusion, TOF, MR angiography Large vessel occlusion (LVO) in stroke patients is mostly detected using deep-learning-based automated methods on CT angiography but there has been no report on such methods using time-of-flight magnetic resonance angiography (TOF-MRA). Our study includes 460 stroke patients with 230 LVO-positive cases. The first step was vessel segmentation, and the output mask was used for the LVO detection. Both steps were deep-learning based using TOF-MRA. Our model successfully detected 95% LVO-positive and 92% LVO-negative patients. The high detection rate and short processing time (< 60 seconds) suggested that our model is highly adequate in a clinical emergency context. |
| 15:45 |
0700.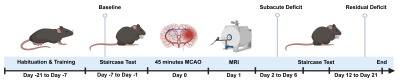 |
Machine learning-based prediction of stroke outcome in mice
from MRI and behavioral testing
Philipp Boehm-Sturm1,2,3,
Felix Knab1,2,
Stefan Paul Koch1,2,3,
Sebastian Major1,2,
Tracy D. Farr1,2,3,4,
Susanne Mueller1,2,3,
Philipp Euskirchen1,
Moritz Eggers1,2,
Melanie Kuffner1,2,
Josefine Walter1,2,5,
Jens P. Dreier1,2,6,7,
Matthias Endres1,2,6,8,9,10,
Ulrich Dirnagl1,2,6,8,9,10,
Nikolaus Wenger1,2,6,11,
Christian J. Hoffmann1,2,11,
and Christoph Harms1,2,6,8,9
1Klinik und Hochschulambulanz für Neurologie, Department of Experimental Neurology, Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany, 2Center for Stroke Research Berlin, Charité-Universitätsmedizin Berlin, Berlin, Germany, 3NeuroCure Cluster of Excellence and Charité Core Facility 7T Experimental MRIs, Charité-Universitätsmedizin Berlin, Berlin, Germany, 4School of Life Sciences, University of Nottingham, Berlin, Germany, 5QUEST Center for Transforming Biomedical Research, Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Berlin, Germany, 6Einstein Center for Neuroscience, Berlin, Germany, 7Bernstein Center for Computational Neuroscience, Berlin, Germany, 8German Center for Cardiovascular Research (DZHK), partner site Berlin, Berlin, Germany, 9NeuroCure Clinical Research Center, Charité-Universitätsmedizin Berlin, Berlin, Germany, 10German Center for Neurodegenerative Diseases (DZNE), Berlin, Germany, 11Berlin Institute of Health (BIH), Berlin, Germany Keywords: Stroke, Animals, Machine learning, Mice, MCAO Prediction of motor-functional outcome in mice could potentially guide researchers in their treatment decisions in preclinical stroke intervention studies and support outcome-dependent stratifications. We pooled 13 studies in which mice underwent identical MRI and behavioral testing protocols. In this large cohort of mice (n=148), we developed and compared machine learning-based predictors of post-stroke recovery. |
| 15:45 |
0701.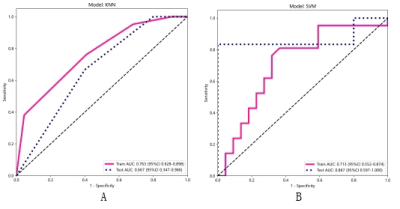 |
Identification of high-risk basilar artery plaque with
HR-VWI-based radiomics and machine learning
Juan Bai1,
Jiang Wu1,
Ning Li1,
Xuan Li1,
and Kaiyu Wang2
1Shanxi Cardiovascular Hospital, Taiyuan, China, 2MR Research China,GE Healthcare, Beijing, China Keywords: Blood vessels, Atherosclerosis, Radiomics;Machine Learning The stability of plaques is the key cause of cerebral ischemic in patients with basilar atherosclerosis. High-resolution vessel wall imaging (HR-VWI) is a useful technique for study of plaques. Radiomics are widely used in recently years to discover the radiographic information of diseases and to improve diagnostic performance. In this study, we constructed six machine learning models based on radiomics features from HR-VWI to predict the stability of plaques of the basilar artery, finding support vector machine has the best performance. |
| 15:45 |
0702.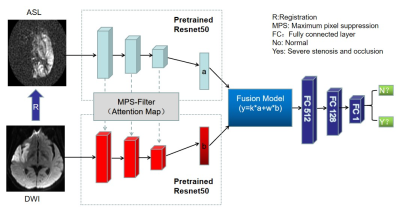 |
Deep Learning Based Algorithm to Identify Large Vessel
Stenosis and Occlusion on Contrast Agent-free Magnetic
Resonance Imaging
Di Wu1,
Mengzhou Sun2,
Yi Li3,
Xiaoyun Liang3,
Feng Huang3,
and Wenzhen Zhu1
1Radiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, 2Neusoft Medical Systems Co. Ltd, Shenyang, Liaoning, China, Beijing, China, 3Neusoft Medical Systems Co. Ltd, Shenyang, Liaoning, China, Shanghai, China Keywords: Stroke, Machine Learning/Artificial Intelligence Large vessel occlusion detection based on clinical scales is of low sensitivity and that based on CTA needs contrast agent exposure. This study aims to develop a deep learning (DL) algorithm for detecting intracranial large vessel steno-occlusion on contrast agent-free MR techniques including DWI and ASL. The accuracy of the DL algorithm was 88.2% with a sensitivity of 88.0%, comparable to CTA-based DL algorithms with sensitivity ranging from 67% to 94%. The MR-based DL algorithm is feasible to accurately detect intracranial large vessel steno-occlusion without intervention, radiation exposure and contrast agent, which could optimize stroke workflow and guide clinical decision-making. |
| 15:45 |
0703.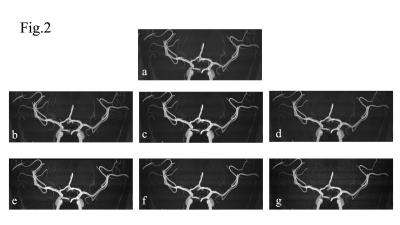 |
High Resolution TOF-MRA Using SmartSpeed-AI for the
Visualization of Lenticulostriate Arteries at 3.0 T: a
Preliminary Study
Yuya Hirano1,
Noriyuki Fujima2,
Kinya Ishizaka1,
Takuya Aoike1,
Jihun Kwon3,
Masami Yoneyama3,
and Kohsuke Kudo4,5
1Department of Radiological Technology, Hokkaido University Hospital, Sappro, Japan, 2Department of Diagnostic and Interventional Radiology, Hokkaido University Hospital, Sapporo, Japan, 3Philips Japan, Ltd, Tokyo, Japan, 4Department of Diagnostic Imaging, Hokkaido University Graduate School of Medicine, Sapporo, Japan, 5Global Center for Biomedical Science and Engineering, Faculty of Medicine, Hokkaido University, Sappro, Japan Keywords: Blood vessels, Image Reconstruction SmartSpeed-AI is recently introduced as a physics driven type deep learning-based novel image reconstruction technique. We investigated the utility of SmartSpeed-AI for the better visibility of lenticulostriate artery (LSA) in high spatial resolution TOF-MRA by comparing the compressed-sensing sensitivity-encoding (compressed SENSE) algorithm. Both quantitative and qualitative assessment revealed that the visibility of LSAs were significantly higher under the SmartSpeed-AI reconstruction than compressed SENSE. SmartSpeed-AI can be helpful to provide superb image quality for the depiction of small arteries like LSA in high resolution TOF-MRA. |
| 15:45 |
0704.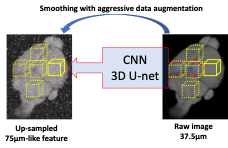 |
Super-resolution neural network-driven mouse cerebrovascular
mapping with MION-based MRI
Xiaoqing Alice Zhou1,
David Hike1,
Bei Zhang1,
Zeping Xie1,
Xiaochen Liu1,
Matthew S Rosen1,
Juan Eugenio Iglesias1,2,3,
and Xin Yu*1
1Massachusetts General Hospital, Boston, MA, United States, 2University College London, London, United Kingdom, 3Massachusetts Institute of Technology, Boston, MA, United States Keywords: Blood vessels, Alzheimer's Disease High-resolution cerebrovascular mapping is crucial for the understanding of brain function. While the existing invasive imaging modalities map brain vasculature at the microscopic scale, they are mostly restricted to the cortical surface in small animals. The non-invasive penetrating cerebrovascular mapping for larger animals remains the privilege of MRI. To achieve high spatial resolution with MRI, we trained 3D U-net to super-resolve images from 75µm to 37.5µm isotropic resolution. Our results show a significantly improved segmented micro-vessel network and offer a powerful tool to identify the anatomical distribution of the microvessels in normal and degenerative brains longitudinally. |
| 15:45 |
0705.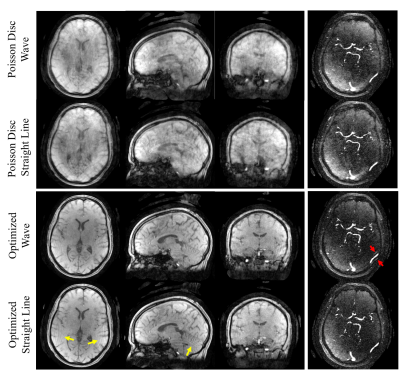 |
Machine Learned Wave Encoded Neurovascular 4D Flow
Chenwei Tang1,
Leonardo A Rivera-Rivera1,2,
Laura B Eisenmenger3,
and Kevin M Johnson1,3
1Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Medicine, University of Wisconsin-Madison, Madison, WI, United States, 3Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States Keywords: Blood vessels, Velocity & Flow Keywords: Wave Encoding, Trajectory Optimization, 4D Flow Non-Cartesian sampling is often required for 4D Flow imaging because of more efficient sampling. Due to the heuristic nature of the optimization of such trajectories, we propose to parameterize and optimize wave encoded 3D Cartesian sampling using a gradient descent algorithm in a data-driven way. We demonstrate the feasibility of our framework in learning the sampling patterns and the wave parameters and providing high image quality for highly accelerated scans in digital phantoms, phantoms and in vivo with phase contrast. |
| 15:45 |
0706.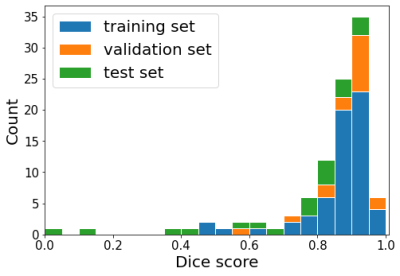 |
Deep learning-based segmentations challenge established link
between stroke volume and functional outcome after
thrombectomy
Ingrid Digernes1,
Martin Soria Røvang1,
Terje Nome2,
Cecilie Nome3,
Thor Håkon Skattør2,
Brian Anthony Enriquez3,
Bradley J MacIntosh1,
Anne Hege Aamodt3,
and Atle Bjørnerud1
1Computational Radiology and Artificial Intelligence, Oslo University Hospital, Oslo, Norway, 2Radiology and Nuclear Medicine, Oslo University Hospital, Oslo, Norway, 3Department of Neurology, Oslo University Hospital, Oslo, Norway Keywords: Stroke, Machine Learning/Artificial Intelligence, segmentation, functional outcome, thrombectomy Using a standard 3D nnUNET model that was pretrained to segment WMH on FLAIR, we obtained good stroke lesion segmentation accuracy (median Dice = 0.80) when the model was re-trained to segment the stroke lesion on DWI in a smaller stroke sample (N = 82). Applying the segmentation model to DWIs from 307 thrombectomy patients, we found no association between pre-treatment lesion volume and functional outcome at 90 days after thrombectomy. Our results indicate that the established assumption of lesion size being a strong predictor of functional outcome should be investigated further for patients receiving mechanical thrombectomy. |
| 15:45 |
0707.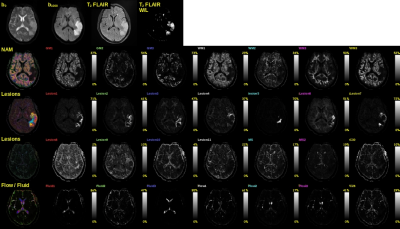 |
The Fuzzy MAD Stroke Conjecture, using Fuzzy C Means to
classify Multimodal Apparent Diffusion for stroke
stratification
Frederick C. Damen1,
Changliang Su2,
Thomas Anderson1,
Tibor Valyi-Nagy3,
Leon Tai4,
Rifeng Jiang5,
and Kejia Cai1,6
1Radiology, University of Illinois at Chicago, Chicago, IL, United States, 2Medical Imaging, Sun Yat-sen University Cancer Center, Guangzhou, China, 3Pathology, University of Illinois at Chicago, Chicago, IL, United States, 4Anatomy and Cell Biology, University of Illinois at Chicago, Chicago, IL, United States, 5Radiology, Fujian Medical University Union Hospital, Fuzhou, China, 6Bioengineering, University of Illinois at Chicago, Chicago, IL, United States Keywords: Stroke, Diffusion/other diffusion imaging techniques The ischemic stroke cascade is a complex menagerie of processes causing disruption of neuronal function and willful and/or reactive breakdown of the neural vascular unit (NVU), and, prevails to recovery, repair, or patient demise. To better understand the underpinnings of the stroke cascade we applied our Multimodal Apparent Diffusion (MAD) method to multi b‑value diffusion weighted magnetic resonance imaging, up to b‑value of 10K s/mm2, on 41 consecutive stroke patients. Using Fuzzy C Means we were able to discern 13 normal appearing tissue types and 16 lesion types. There are several findings that should be contemplated in current clinical imaging. |
| 15:45 |
0708.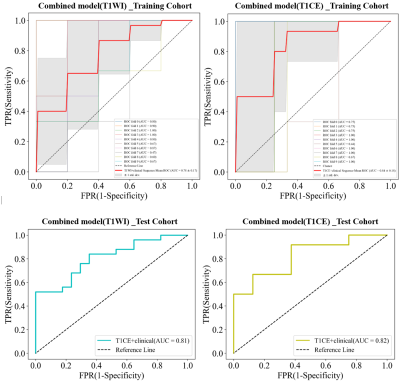 |
Prediction of Ischemic Stroke Based on Carotid Plaque
VW-HRMRI Radiomics
Na Han1,
Wanjun Hu1,
Yurong Ma1,
Laiyang Ma1,
Yu Zheng1,
Jing Zhang1,
and Kai Ai2
1Department of Magnetic Resonance, Lanzhou University Second Hospital, Lanzhou, China, 2Philips Healthcare, Xi’an, China Keywords: Stroke, Radiomics, Carotid Plaque, Vessel Wall High-Resolution MRI The study of the relationship between carotid atherosclerotic plaque and ischemic stroke must go beyond the assessment of the basic imaging characteristics of plaque or the degree of vascular stenosis, and should adopt a new model which carotid plaque imaging combined with artificial intelligence. This study established a radiomics model of carotid plaque based on vessel wall high-resolution MRI (VW-HRMRI) to accurately predict the risk of ischemic stroke. Our study showed that the radiomics_T1CE model and combined_T1CE model outperformed other models, whereas the combined model showed no significant difference with the radiomics model. |
| 15:45 |
0709.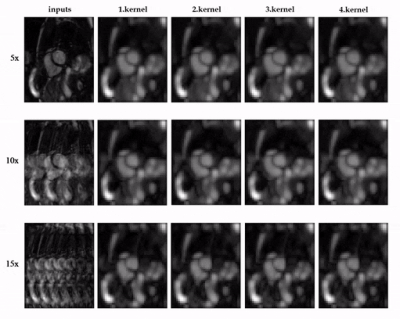 |
Self-supervised contrastive learning for MR image
reconstruction of cardiac CINE on accelerated cohorts
Siying Xu1,
Marcel Früh1,
Kerstin Hammernik2,3,
Sergios Gatidis1,4,
and Thomas Küstner1
1Medical Image and Data Analysis (MIDAS.lab), Department of Diagnostic and Interventional Radiology, University Hospital of Tuebingen, Tuebingen, Germany, 2Lab for AI in Medcine, Technical University of Munich, Munich, Germany, 3Department of Computing, Imperial College London, London, United Kingdom, 4Max Planck Institute for Intelligent Systems, Tuebingen, Germany Keywords: Image Reconstruction, Machine Learning/Artificial Intelligence, Self-Supervised Learning, Image Reconstruction, Heart Existing methods for deep-learning reconstruction require abundant fully-sampled images as labels, which is challenging or impractical in practice. To address this issue, we propose a self-supervised learning (SSL) approach that can be trained on subsampled data, avoiding the need for fully-sampled datasets. Specifically, we pre-train a feature extractor by contrastive learning as the first step. In the second step, the pre-trained feature extractor assists the self-supervised network during reconstruction by feature embedding. Results indicate that the proposed SSL method can effectively reconstruct cardiac CINE images without fully-sampled data. It outperforms existing SSL networks and shows comparable results to supervised learning. |
| 15:45 |
0710.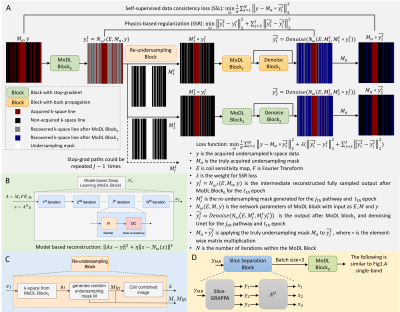 |
Self-supervised Learning with Self-supervised Regularization
Reconstruction for Accelerated Single- and Multiband
Myocardial Perfusion MRI
Changyu Sun1,2,
Kenneth Bilchick3,
Michael Salerno4,
and Talissa A. Altes2
1Biomedical, Biological and Chemical Engineering, University of Missouri Columbia, Columbia, MO, United States, 2Radiology, University of Missouri Columbia, Columbia, MO, United States, 3Department of Medicine, University of Virginia Health System, Charlottesville, VA, United States, 4Department of Medicine, Stanford University, Stanford, CA, United States Keywords: Image Reconstruction, Heart, Self-supervised Learning Physics-guided self-supervised learning (PG-SSL) of MRI reconstruction may provide high spatiotemporal fidelity and fast reconstruction of highly accelerated first-pass myocardial perfusion MRI without ground truth. We sought to develop a SSL model with self-supervised regularization (SSR) using Siamese architecture with stop gradient and re-undersampling block to generate physics-based data augmentation and regularization. PG unrolled network was used as the sub-network in Siamese structure. Self-supervised learning with self-supervised regularization (SSLR) outperformed low rank plus sparse on retrospective rate-8 undersampling single-band data and showed improved SNR and temporal fidelity on prospective multiband whole-heart coverage high resolution perfusion imaging. |
| 15:45 |
0711.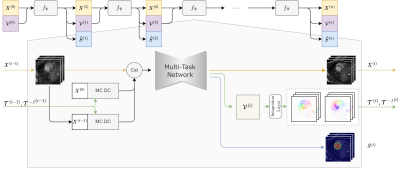 |
A unified deep learning model for simultaneous cardiac cine
MRI reconstruction, motion estimation and segmentation.
Pengfang Qian1,2,
Junwei Yang3,
Zijian Zhou1,2,
Peng Hu1,2,
and Haikun Qi1,2
1School of Biomedical Engineering, ShanghaiTech University, Shanghai, China, 2Shanghai Clinical Research and Trial Center, Shanghai, China, 3Department of Computer Science and Technology, University of Cambridge, Cambridge, United Kingdom Keywords: Image Reconstruction, Machine Learning/Artificial Intelligence Various deep learning methods have been proposed for cardiac cine MRI, including accelerated image reconstruction, cardiac motion estimation and segmentation, which are traditionally considered as separate tasks without exploiting the inter-task correlation. In this study, we propose a unified deep learning model to perform accelerated cine image reconstruction, motion estimation and segmentation simultaneously in an iterative framework, where correlations between tasks are exploited by compensating motion in reconstruction, semi-supervising segmentation using pseudo-labels generated by motion and improving motion estimation using intermediately reconstructed images. Experiment results show that the multi-task model outperformed single-task networks. |
| 15:45 |
0712.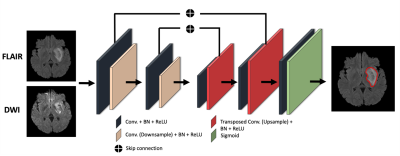 |
Deep learning-based stroke segmentation and patient outcome
prediction
Hae Sol Moon1,
Lindsay Heffron2,
Ali Mahzarnia3,
Barnabas Obeng-Gyasi3,
Matthew Holbrook3,
Cristian T. Badea1,3,
Wuwei Feng4,
and Alexandra Badea1,3,4,5
1Biomedical Engineering, Duke University, Durham, NC, United States, 2Orthopaedic Surgery, Duke University School of Medicine, Durham, NC, United States, 3Radiology, Duke University School of Medicine, Durham, NC, United States, 4Neurology, Duke University School of Medicine, Durham, NC, United States, 5Brain Imaging and Analysis Center, Duke University School of Medicine, Durham, NC, United States Keywords: Multimodal, Data Analysis, Deep learning; Segmentation; Lesion Load; Stroke We compared the ability of 2D and 3D U-Net Convolutional Neural Network (CNN) architectures to segment ischemic stroke lesions and predict patient outcome using single-contrast (DWI) and dual-contrast images (T2w FLAIR and DWI). The predicted lesion segmentation metrics and location relative to corticospinal tract correlated with post-stroke patient outcome measured by National Institutes of Health Stroke Scale (NIHSS). The 2D multi-modal CNN achieved the best results with mean Dice of 0.74. The highest correlation was for weighted-lesion load with both baseline and 90-days NIHSS (80%, p<0.001). Our results support that multi-contrast MR helps automate lesion segmentation and predict post-stroke outcomes. |
| 15:45 |
0713.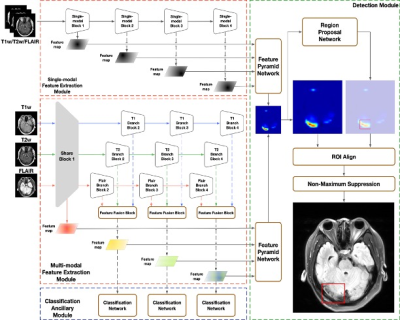 |
CVT Detection with Routine Brain MRI Sequences by
Multi-Modal Multi-Task Deep Learning algorithm
Xiaoxu Yang1,
Pengxin Yu2,
Haoyue Zhang2,
Rongguo Zhang2,
Yuehong Liu1,
Xiuqin Jia1,
Penghui Sun1,
Xin Liu1,
Xunming Ji3,
Qi Yang1,
and Chen Zhang4
1Beijing Chaoyang Hospital, Beijing, China, 2Infervision Medical Technology Co., Ltd, Beijing, China, Beijing, China, 3Beijing Xuanwu Hospital, Beijing, China, 4MR Scientific Marketing, Siemens Healthineers, Beijing, China, Beijing, China Keywords: Stroke, Brain A deep learning algorithm for detecting cerebral venous thrombosis using routine brain MRI achieved higher patient-level sensitivity than radiologists and reduced the number of overlooked thrombosed segments. The proposed deep learning (DL) algorithm achieved area under the receiver operating characteristic curve of 0.96 for detecting patient with cerebral venous thrombosis. The sensitivity of DL algorithm was higher than that of radiologists and obtained high specificity on patient-level.Compared to radiologists, DL algorithm found more thrombosed segments, indicating greater sensitivity and a sufficient specificity at the segment-level. |
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.