2026 ISMRM Abstract Review Instructions
Online Review & Scoring Deadline:
Please read all instructions carefully before you begin.
Thank you for agreeing to review abstracts for the 2026 ISMRM Annual Meeting. Your role as a reviewer is integral to the success of the scientific program, and your efforts are greatly appreciated. Your reviews will assist the Annual Meeting Program Committee (AMPC) as they construct the scientific program for the Annual Meeting. Please read the following instructions before reviewing your assigned abstracts. Finally, please note that the content and scoring of all abstracts is strictly confidential.
Starting the Abstract Review
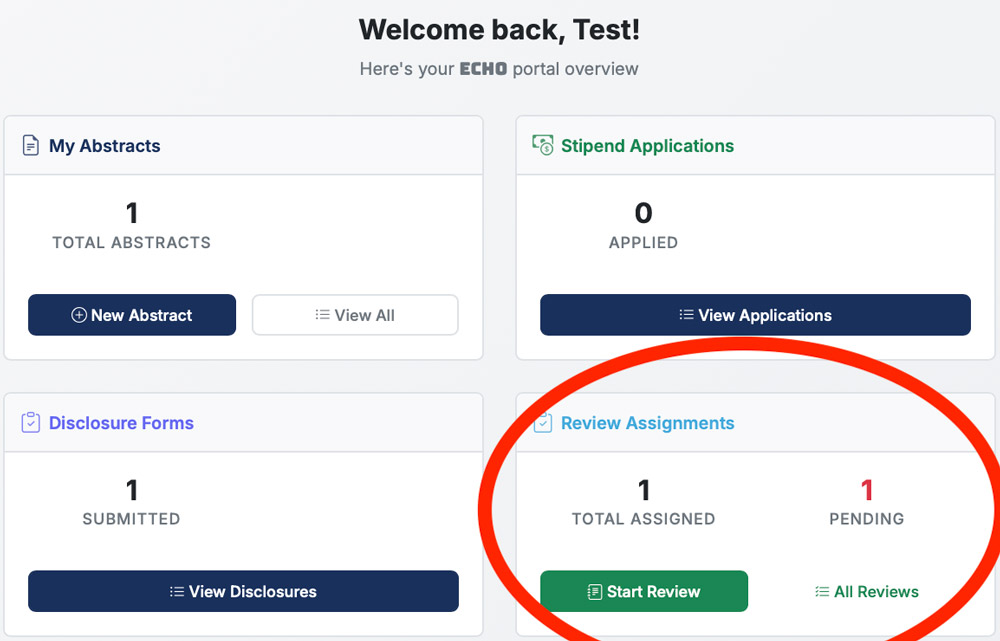
Abstracts are reviewed using the ECHO platform. To begin, please go to echo.ismrm.org and log in to your account. You can access your review page using 'My Reviews' at the top or the 'Review Assignments' panel on your ECHO homepage.
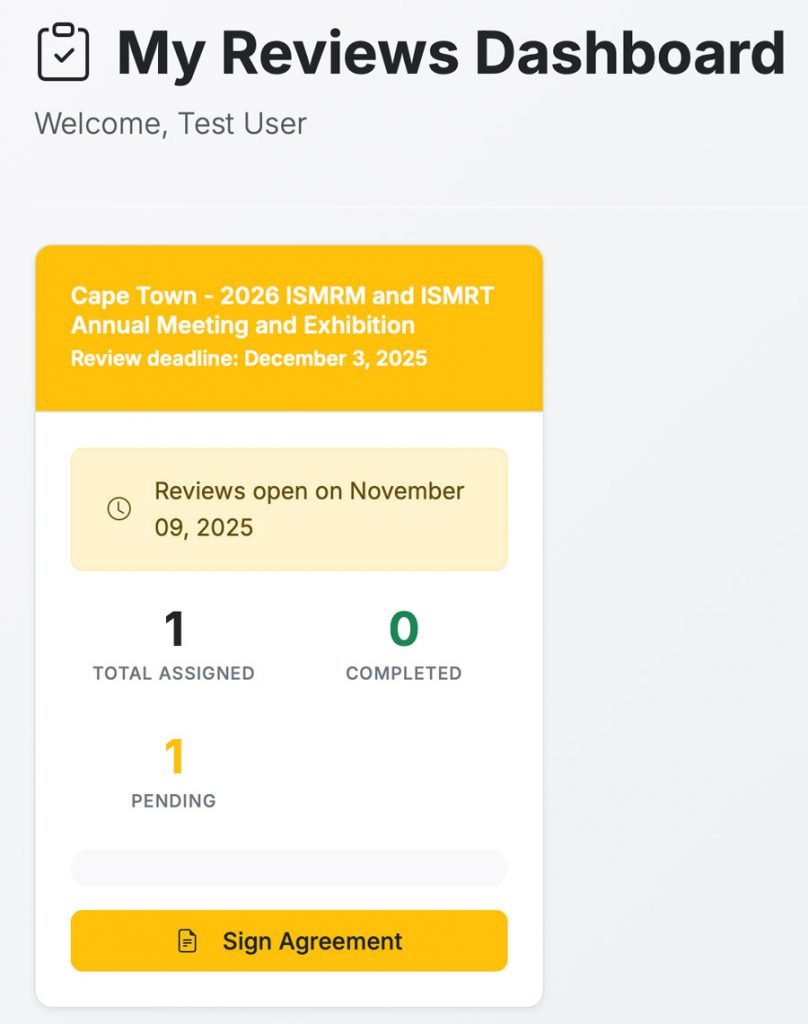
This will direct you to your Reviews dashboard where you can start the Review process.
Reviewer Agreement
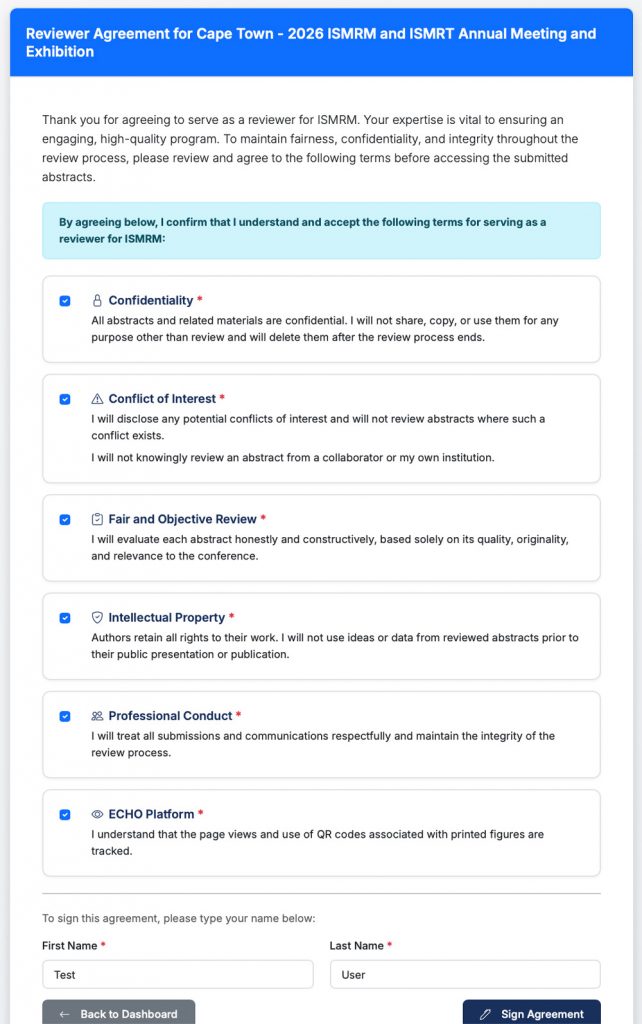
Your next step is to sign the Reviewer Agreement. Authors invest an enormous amount of time, effort, and resources to generate their research. As reviewers, you have a responsibility to take this process seriously, and you are serving as representatives of the ISMRM. By agreeing to review abstracts, you are agreeing to abide by the following principles:
- Confidentiality: The content of every abstract is strictly confidential until it is published in the Proceedings of the Annual Meeting. Strict adherence to confidentiality has many implications, including grant funding, intellectual property, and publications. Do not share any aspect of your assigned abstracts or your scoring with anyone. Please delete any download copies at the end of your review process.
- Conflict of Interest: Please disclose any conflicts of interest and do not review any abstracts where such a conflict exists. Please do not knowingly review an abstract from a collaborator or your own institution. Additionally, Reviewers are required to disclose all relevant financial disclosures involving any commercial interest.
- Fair and Objective Review: Please remember to evaluate the science only. We do not reveal authors, institutions, or acknowledgements as a way to help you, the reviewer, focus on the science and avoid unconscious bias. However, for some submissions, you may recognize the authors or institution based on your familiarity with specialized research equipment or continuation of prior work. We ask that you set that aside and evaluate the science alone.
- Intellectual Property: Authors retain all rights to their work.
- Professional Conduct: Please treat submissions and communications respectfully and maintain the integrity of the review process.
- ECHO Platform: The page views and use of QR codes associated with printed figures are tracked by ECHO.
Please sign the agreement to proceed to reviewing abstracts.
How are Abstracts Assigned?
When signing up to review abstracts, reviewers self-select their expertise across a range of skill sets. After submission, abstracts are assessed and scored across these same topic areas. Then, the abstracts and reviewers are matched based on these skills or topics including factors such as reviewer experience and reviewer load (how many abstracts you review). Most reviewers will receive abstracts from across their reported expertise. Please try to score consistently across the abstracts that you review, and avoid scoring one research topic area higher than another.
If you would prefer to receive abstracts on different research topics, please consider the skills that you self-selected and revise these selections in the future.
The Review Interface
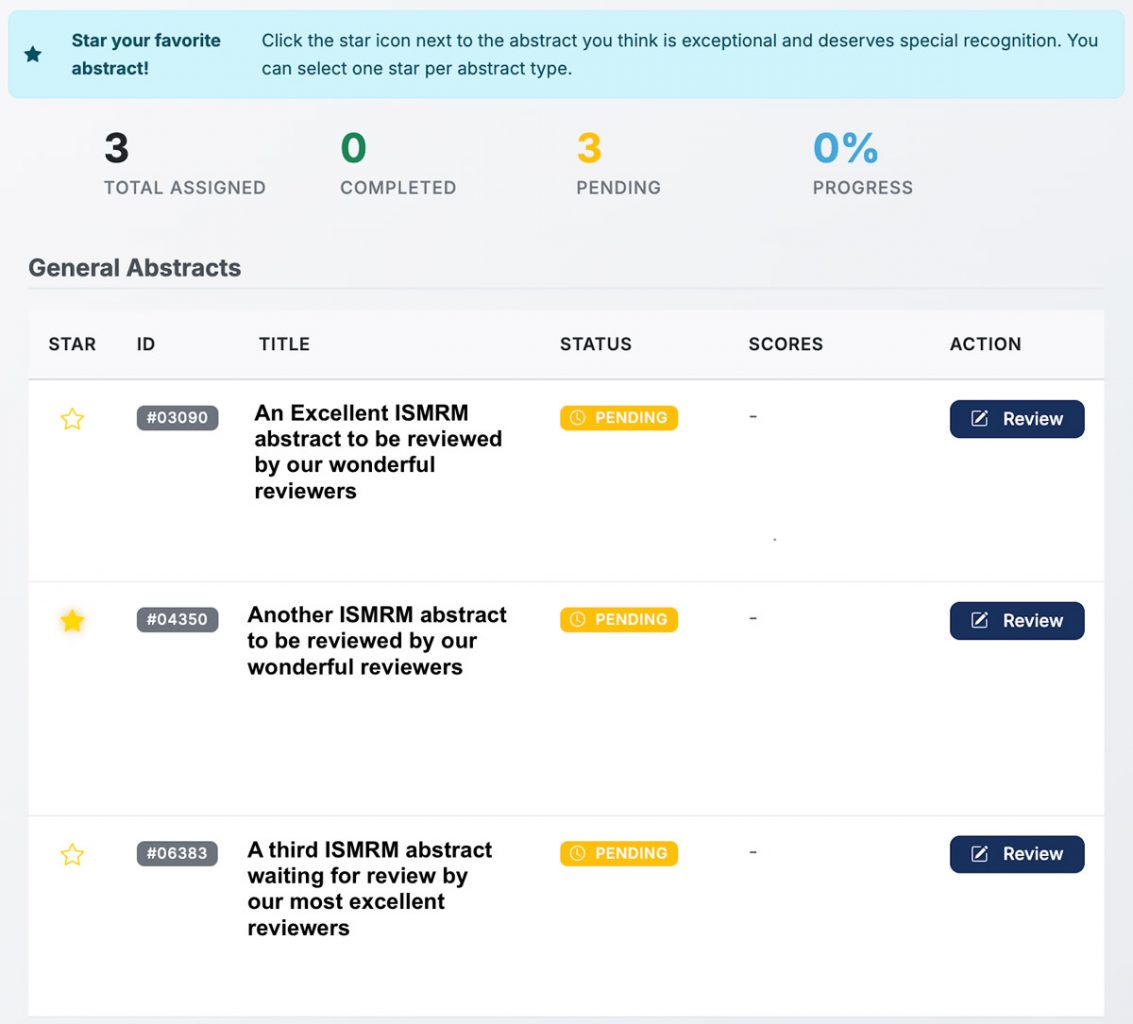
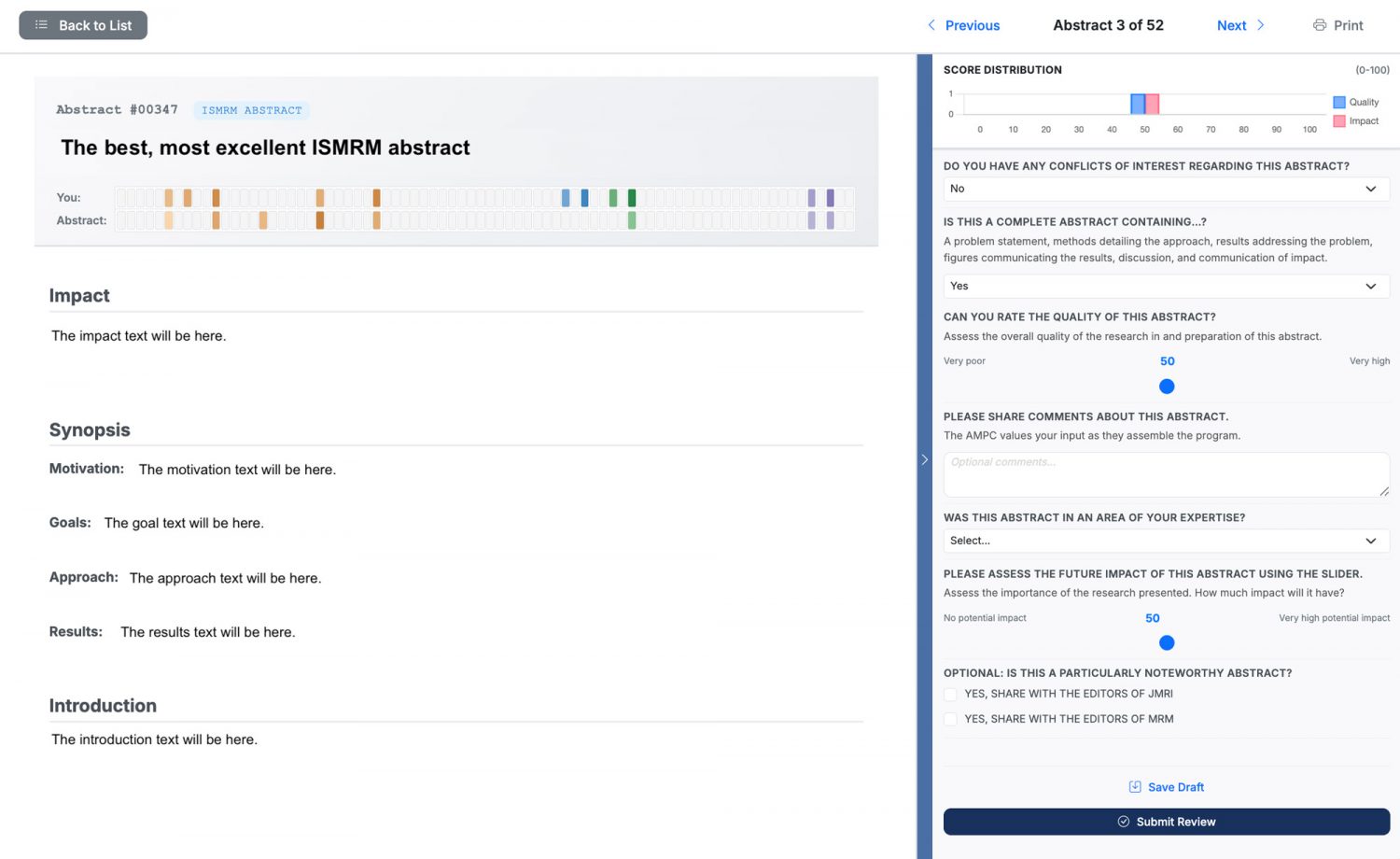
Once you have completed your reviewer agreement and review is open, you can begin reviewing abstracts. From the Review Dashboard, you can see the list of abstracts for you to review. You'll also view scores for any abstracts that you have already reviewed. To begin, select the Review action.
When reviewing an abstract, the abstract will be on the left side, and the review panel will be on the right side. You can use the blue vertical bar to view or hide the review panel. Details on the review questions are below in 'How to Review.' When you have completed your review, please select 'Submit Review.' After submitting your review, if needed, you can 'Edit Review' to modify your evaluation of the abstract.
Below the abstract title, you will see two bars with colored rectangles. The top bar, labeled 'You', reflects the skills and scores that you selected when signing up to Review. The bottom bar, labeled 'Abstract', shows the skills and scores for this abstract. These heatmaps illustrate why this abstract was assigned to you.
If you would like to batch download abstracts, from the Review Dashboard, click 'Batch Download' on the right side. Then, be sure to click the 'Print' button on the next page. This will render the abstracts for a batch download to pdf.
Finally, we would like to know which of the abstracts that you reviewed was the best. On the Review dashboard (where you can view all abstracts assigned to you for review). Please indicate your favorite abstract with a star.
How to Review
Your reviews will assist the Annual Meeting Program Committee (AMPC) as they construct the scientific program for the Annual Meeting. The AMPC greatly appreciates your assessment of the quality of the submitted abstracts. Please help us identify the high quality work that we would all like to see at the meeting.
These are general review instructions, focused on the Standard ISMRM abstract. For details about reviewing Registered Abstracts and MRI in Clinical Practice Abstracts, please see below.
We'll step through the Review process, considering each question:
CONFLICT OF INTEREST: First, please evaluate if you have a Conflict of Interest with the abstract. Conflicts can arise if you are a co-author on the abstract, a collaborator of the author, or at the same institution. A conflict also exists if you hold patents directly related to the research, or there is any other reason generally considered a conflict. Blinded review can make it difficult to identify all conflicts, but we ask you to do your best. If you recognize this work and have a potential conflict, please select 'yes' and then 'Save Review.' Your 'review' of this abstract is complete.
OVERALL ASSESSMENT: Then, please consider the abstract as a whole. Does this abstract contain:
- A problem statement?
- Methods detailing the approach to answer the stated problem?
- Results addressing the problem?
- Figures communicating the results?
- A discussion of the results?
- Communication of the impact of the work?
A few additional considerations as you review:
- Duplication of Abstracts: Clear duplication or strongly overlapping abstracts is grounds for rejection of one or both abstracts. Please score duplicated abstracts as if they were independent, and then note any duplication (provide abstract numbers) in the comment section. Also, abstracts that are essentially the same as previously published journal articles should be identified. Please note this kind of duplication in the comment section as well, including a brief citation if possible.
- Blinded Review: Abstracts are blinded by authors and institutions. If an author inadvertently identifies himself or herself by name or affiliation, you may choose to review or not review, whichever you consider most appropriate. If inadvertent disclosure occurs, please note this in the Comments section and inform the ISMRM Office as quickly as possible, so this disclosure can be corrected. (If authors cite their own work, you do not need to comment on that.)
- Standards Involving Recommendations for Clinical Care:
- All recommendations involving clinical medicine must be based on evidence that is accepted within the profession of medicine as adequate justification for their indications and contraindications in the care of patients.
- Basic science / engineering research referred to, reported or used in support or justification of any patient care recommendation must conform to generally accepted standards of experimental design, data collection and analysis.
- Please note any deviations from these principles in the Comments section.
Considering these points will help you assess the quality of the work.
QUALITY: Please assess the quality of the research (including study design, appropriateness of the research goal/question, rigor of the data analysis) AND the clarity with which the work is presented. Accepted abstracts will be published and referenced. For this reason, abstracts should be of high quality. Poorly prepared abstracts with spelling errors, confusing formatting, and poor grammar should be scored accordingly. Please use the entire 0-100 scoring range as much as possible.
COMMENTS: We ask that you provide brief comments whenever you can. Taking 30 seconds to write a comment is very helpful to the program committee. If you believe the abstract is a duplicate of another abstract or a published paper, please score the abstract, and identify the duplicate in the comments. Please note, third party plug-ins may cause the comment box to move on the page when trying to type. Disabling third party plug-ins has resolved this issue.
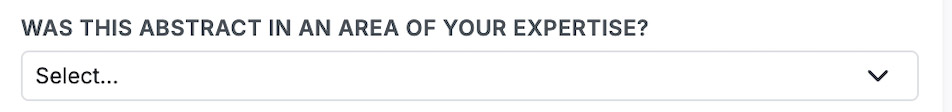
REVIEWER EXPERTISE: Please evaluate whether or not the abstract was in an area of your expertise. It is possible (and understandable) that some of your assigned abstracts are beyond your ability to provide an informed evaluation. You must use your judgment here – if possible please review the abstract. However, if you feel unqualified to assess an abstract fairly, please check “yes” under Conflict of Interest, make a note in the comment section that the abstract is beyond your expertise, and let us know by responding here.
IMPACT: Please assess the future impact of this work. Please use the entire 0-100 scoring range as much as possible.
JOURNAL HIGHLIGHT: In an attempt to streamline submission, review, and publication of strong abstracts to our journals, we invite you to select 2 to 3 of the abstracts you have reviewed as being of sufficiently high quality to warrant notification to the journal editors. This information will be sent to the MRM and JMRI editors who will have the discretion to use this information as they see fit.
For each abstract you would like to see as a journal paper you should:
(1) recommend which journal,
(2) specify your willingness to review this paper, and
(3) offer high-level comments to the authors, if you have any, to guide them in preparation of an article.
If you answer that you recommend this as a journal submission, additional questions will appear as shown.
All subsequent processes using this information (invitations, review, blindedness of review, and decisions) are entirely at the discretion of the journal editors. Reviewer names are kept confidential by the ISMRM and the journals. Note that information in response to this question will NOT be shared with the Annual Meeting Program Committee in final selection.
Again, the goal of this option is to not only streamline submission, review, and publication of strong abstracts, but also incentivize authors of the best abstracts to write them up as full papers and to help feature our best ISMRM meeting content in our own journals.
Reviewing Registered Abstracts
If you've been asked to review Registered Abstracts, these will appear on your 'My Reviews' page under the heading 'Registered Abstracts.'
What is a registered abstract?
A Registered Abstract is an abstract where research methods are submitted and assessed by reviewers before the results are known.
How is a Registered Abstract reviewed?
Abstracts are evaluated on their overall quality, similar to conventional abstracts. For any questions about overall quality or Conflicts of Interest, please see the General abstract review directions. Registered abstracts are evaluated as to whether or not they meet the requirements of a Registered Abstract, and the reviewer is asked to evaluate the impact of the abstract.
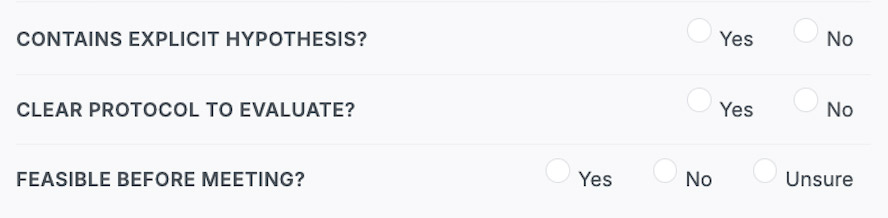
Registered Abstracts have to achieve three minimum requirements for acceptance:
- The abstract has to include an explicit testable hypothesis.
- The abstract has included an explicit protocol for the evaluation of their hypothesis.
- The abstract has to show feasibility to gather and analyze data in time for the annual meeting.
How to score a Registered Abstract on Quality
The quality score should reflect both the quality of the research (including study design, appropriateness of the research goal/question, rigor of the statistical & data analysis) AND the clarity with which the work is presented (easy to understand and well written text as well as clear and well labeled figures).
Registered Abstracts should use future tense to describe all work that is not yet completed.
Experiments and data analyses must be well defined (all relevant conditions and variables clearly listed) to reduce researcher degrees of freedom after data has been collected. Additionally, reasons for design choices need to be justified (e.g., based on pilot data or previous literature).
How to score a Registered Abstract on Impact
For Registered Abstracts, impact is assessed on the importance of answering the research question. For example, if the answer to “is method A more motion robust than method B?” would allow for clinical translation of the more motion robust method, that could warrant a high impact score. Conversely, an abstract regarding fine tuning of a parameter for a method that is far from clinical translation would have low impact. Impact could also come from the ability to definitively answer a question. For example, a large well-powered study on the effectiveness of some method would yield a higher impact score than a smaller study. In summary, impact is the degree to which the work will influence the field, including how likely the work is to change scientific discovery, clinical practice or further technique development and capability.
How to check a Registered Abstract for minimum requirements
Reviewers of Registered Abstracts are asked to confirm whether minimum requirements have been met.
For a Registered Abstract to be accepted, reviewers need to be able to identify an explicit hypothesis. The reviewer will have to answer yes/no to the question “does the abstract contain an explicit testable hypothesis?” Then, the reviewer will need to answer whether or not the abstract includes an explicit protocol for the evaluation of their hypothesis.
Finally, to avoid accepting abstracts that fail to submit results in time for the meeting, reviewers will comment on the ability to complete the study in time for the annual meeting. When making this evaluation, reviewers should consider the proposed experiments and whether data collection has started (and the data is just not yet analyzed) and whether the data collection is easy (e.g., phantom data) or hard (e.g., specific patient populations).
Reviewing MRI in Clinical Practice Abstracts
If you've been asked to review MRI in Clinical Practice Abstracts, these will appear on your 'My Reviews' page under the heading 'Clinical Abstracts.'
MRI in Clinical Practice abstracts should present the added value of MRI in impacting patient care. Authors were encouraged to construct the abstract as a narrative of the patient's clinical history including unique medical and social factors, as appropriate. An analysis of a series of cases is also appropriate for this category. The abstract must include specifics of the utilization, technical specifications, and contribution of magnetic resonance imaging (MRI) in the management of the patient's clinical presentation.
These abstracts should be evaluated based on the added value of MRI in impacting patient care, particularly where MRI provided unique diagnostic or management insights.
Please evaluate the quality of the abstract including how it presents the added value of MRI in impacting patient care. In addition to the questions presented for General Abstract review, you will answer explicitly if the abstract presents the added value of MRI. Comments to the AMPC will be very helpful as we plan how to incorporate these abstracts in the scientific program.
The requirements for MRI in Clinical Practice abstracts are:
- Title: Suggested format: “MRI in Clinical Practice: Diagnosis of …” OR “MRI in Clinical Practice: Management of …”
- Impact: A 40-word Impact statement should state the impact of MRI: how it changed diagnostic certainty, management, or patient outcome. The impact statement will be provided alongside the Preview figure as a teaser in the meeting program.
- Synopsis: A 100-word unstructured Synopsis should be provided to summarize the report.
- Clinical Presentation and Assessment
- Brief report of patient presentation, physical exam findings, and relevant laboratory tests. No identifiers may be included.
- Describe imaging tests, including MRI, used to work-up the patient. Highlight aspects (e.g., magnet strength, pulse sequence and key parameters, contrast agent) of the MRI technology.
- Diagnosis and Treatment: Report the final diagnosis and how the imaging diagnosis was confirmed (e.g., biopsy, follow-up imaging, combination of imaging and lab tests) and treated.
- Significance: State how MRI was applied in a novel or unique way to address the clinical question and impact patient diagnosis or management. Emphasize the impact of MRI: how it changed diagnostic certainty, management, or patient outcome.
- Key Points: List at least 3 key points about the disease and MR imaging manifestation or treatment impact relevant for clinical practice.
- Figures: Up to 5 images with figure legend. Please use arrows or labels to highlight critical features.
- References: Please appropriately cite relevant previous work including background and methodological information.
Staff Assistance
Rhiannon Pinson, Director of Education: rhiannon@ismrm.org
Melissa Simcox, Associate Executive Director: melissa.simcox@ismrm.org
Sally Moran, Director of IT & Web: sally@ismrm.org
Thank you for your efforts to help make the 2026 ISMRM Annual Meeting a success!