Frequency Drift in MR Spectroscopy: An 87-scanner 3T Phantom Study
Steve C.N. Hui1,2, Mark Mikkelsen1,2, Helge J. Zöllner1,2, Vishwadeep Ahluwalia3, Sarael Alcauter4, Laima Baltusis5, Deborah A. Barany6, Laura R. Barlow7, Robert Becker8, Jeffrey I. Berman9, Adam Berrington10, Pallab K. Bhattacharyya11, Jakob Udby Blicher12, Wolfgang Bogner13, Mark S. Brown14, Vince D. Calhoun15, Ryan Castillo16, Kim M. Cecil17, Yeo Bi Choi18, Winnie C.W. Chu19, William T. Clarke20, Alexander R. Craven21, Koen Cuypers22, Michael Dacko23, Camilo de la Fuente-Sandoval24, Patricia Desmond25, Aleksandra Domagalik26, Julien Dumont27, Niall W. Duncan28, Ulrike Dydak29, Katherine Dyke30, David A. Edmondson17, Gabriele Ende8, Lars Ersland31, C. John Evans32, Alan S. R. Fermin33, Antonio Ferretti34, Ariane Fillmer35, Tao Gong36, Ian Greenhouse37, James T. Grist38, Meng Gu39, Ashley D. Harris40, Katarzyna Hat41, Stefanie Heba42, Eva Heckova13, John P. Hegarty II43, Kirstin-Friederike Heise44, Aaron Jacobson45, Jacobus F.A. Jansen46, Christopher W. Jenkins47, Stephen J. Johnston48, Christoph Juchem49, Alayar Kangarlu50, Adam B. Kerr5, Karl Landheer51, Thomas Lange52, Phil Lee53, Swati Rane Levendovszky54, Catherine Limperopoulos55, Feng Liu56, William Lloyd57, David J. Lythgoe58, Maro G. Machizawa59, Erin L. MacMillan7, Richard J. Maddock60, Andrei V. Manzhurtsev61, María L. Martinez-Gudino62, Jack J. Miller63, Heline Mirzakhanian64, Paul G. Mullins65, Jamie Near66, Wibeke Nordhøy67, Georg Oeltzschner1,2, Raul Osorio62, Maria C.G. Otaduy68, Erick H. Pasaye4, Ronald Peeters69, Scott J. Peltier70, Ulrich Pilatus71, Nenad Polomac71, Eric C. Porges72, Subechhya Pradhan55, James Joseph Prisciandaro73, Nick Puts74, Caroline D. Rae75, Francisco Reyes-Madrigal76, Timothy P.L. Roberts9, Caroline E. Robertson77, Muhammad G. Saleh78, Jens T. Rosenberg79, Diana-Georgiana Rotaru58, O'Gorman Tuura L. Ruth80, Kristian Sandberg12, Ryan Sangill81, Keith Schembri82, Anouk Schrantee83, Natalia A. Semenova84, Debra Singel85, Rouslan Sitnikov86, Jolinda Smith87, Yulu Song36, Craig Stark88, Diederick Stoffers89, Stephan P. Swinnen44, Costin Tanase60, Sofie Tapper1,2, Martin Tegenthoff42, Thomas Thiel90, Marc Thioux91, Peter Truong92, Pim van Dijk91, Nolan Vella82, Rishma Vidyasagar93, Andrej Vovk94, Guangbin Wang36, Lars T. Westle67, Timothy K. Wilbur54, William R. Willoughby95, Martin Wilson96, Hans-Jörg Wittsack97, Adam J. Woods98, Yen-Chien Wu99, Junqian Xu100, Maria Yanez Lopez101, David K.W. Yeung19, Qun Zhao102, Xiaopeng Zhou29, Gasper Zupan94, and Richard A.E. Edden1,2
1Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3GSU/GT Center for Advanced Brain Imaging, Georgia Institute of Technology, Atlanta, GA, United States, 4Instituto de Neurobiología, Universidad Nacional Autónoma de México, Queretaro, Mexico, 5Center for Cognitive and Neurobiological Imaging, Stanford University, Stanford, CA, United States, 6Kinesiology, University of Georgia, Athens, GA, United States, 7Department of Radiology, The University of British Columbia, Vancouver, BC, Canada, 8Center for Innovative Psychiatry and Psychotherpay Research, Department Neuroimaging, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany, 9Department of Radiology, Children's Hospital of Philadelphia, Philadelphia, PA, United States, 10Sir Peter Mansfield Imaging Centre, School of Physics and Astronomy, University of Nottingham, Nottingham, United Kingdom, 11Imaging Institute, The Cleveland Clinic, Cleveland, OH, United States, 12Center of Functionally Integrative Neuroscience, Aarhus University, Aarhus, Denmark, 13Department of Biomedical Imaging and Image-guided Therapy, High-Field MR Center, Medical University of Vienna, Vienna, Austria, 14Department of Radiology, University of Colorado Anschutz Medical Campus, Aurora, CO, United States, 15Tri-Institutional Center for Translational Research in Neuroimaging and Data Science(TReNDS), Georgia State University, Georgia Institute of Technology, and Emory University, Atlanta, GA, United States, 16Neuroscience Research AustraliaNeuRA Imaging, Randwick, Australia, 17Department of Radiology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 18Psychological and Brain Sciences, Dartmouth College, Hanover, NH, United States, 19Department of Imaging & Interventional Radiology, The Chinese University of Hong Kong, Hong Kong, Hong Kong, 20Wellcome Centre for Integrative Neuroimaging, NDCN, University of Oxford, Oxford, United Kingdom, 21Department of Biological and Medical Psychology, University of Bergen, Haukeland University Hospital, Bergen, Norway, 22REVAL Rehabilitation Research Institute (REVAL), Hasselt University, Diepenbeek, Belgium, 23Department of Radiology, Medical Physics, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 24Laboratory of Experimental Psychiatry & Neuropsychiatry Department, Instituto Nacional de Neurología y Neurocirugía, Mexico City, Mexico, 25Department of Radiology, University of Melbourne/ Royal Melbourne Hospital, Melbourne, Australia, 26Malopolska Centre of Biotechnology, Jagiellonian University, Krakow, Poland, 27Clinical Imaging Core Facility, CI2C Lille, Lille, France, 28Graduate Institute of Mind, Brain and Consciousness, Taipei Medical University, Taipei, Taiwan, 29School of Health Sciences, Purdue University, West Lafayette, IN, United States, 30School of Psychology, University of Nottingham, Nottingham, United Kingdom, 31Department of Clinical Engineering, University of Bergen, Haukeland University Hospital, Bergen, Norway, 32CUBRIC, Cardiff University, Cardiff, United Kingdom, 33Center for Brain, Mind and Kansei Sciences Research, Hiroshima University, Hiroshima, Japan, 34Neuroscience, Imaging and Clinical Sciences, University "G. d'Annunzio" of Chieti-Pescara, Chieti, Italy, 35Physikalisch-Technische Bundesanstalt (PTB), Braunschweig und Berlin, Germany, 36Department of Imaging and Nuclear Medicine, Shandong Medical Imaging Research Institute, Shandong University, Jinan, China, 37Human Physiology, University of Oregon, Eugene, OR, United States, 38Physiology, Anatomy, and Genetics/ Oxford Centre for Magnetic Resonance, The University of Oxford / Department of Radiology, The Churchill Hospital, The University of Oxford, Oxford, United Kingdom, 39Department of Radiology, Stanford University, Stanford, CA, United States, 40Department of Radiology, University of Calgary, Calgary, AB, Canada, 41Institute of Psychology, Jagiellonian University, Krakow, Poland, 42Department of Neurology, BG University Hospital Bergmannsheil, Bochum, Germany, 43Psychiary & Behavioral Sciences, Stanford University, Stanford, CA, United States, 44Department of Movement Sciences, KU Leuven, Leuven, Belgium, 45Department of Radiology, University of California San Diego, San Diego, CA, United States, 46Department of Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 47CUBRIC, Cardiff university, Cardiff, United Kingdom, 48Psychology Dept. / Clinical Imaging Facility, Swansea University, Swansea, United Kingdom, 49Biomedical Engineering and Radiology, Columbia University, New York City, NY, United States, 50Psychiatry, Columbia University Irving Medical Center/New York State Psychiatric Institute, New York City, NY, United States, 51Biomedical Engineering, Columbia University, New York City, NY, United States, 52Department of Radiology, Medical Physics, University of Freiburg, Freiburg, Germany, 53Department of Radiology, University of Kansas Medical Center, Kansas, KS, United States, 54Department of Radiology, University of Washington, Seattle, WA, United States, 55Developing Brain Institute, Diagnostic Imaging and Radiology, Children's National Hospital, Washington, DC, United States, 56Department of Psychiatry, Columbia University Irving Medical Center/New York State Psychiatric Institute, New York, NY, United States, 57Division of Informatics, Imaging & Data Sciences, University of Manchester, Manchester, United Kingdom, 58Department of Neuroimaging, King's College London, London, United Kingdom, 59Center for Brain, Mind and KANSEI Sciences Research, Hiroshima University, Hiroshima, Japan, 60Psychiatry and Behavioral Sciences, University of California Davis, Imaging Research Center, Davis, CA, United States, 61Department of Radiology, Clinical and Research Institute of Emergency Pediatric Surgery and Trauma, Moscow, Russian Federation, 62Imágenes Cerebrales, Instituto Nacional de Psiquiatría Ramón de la Fuente, Mexico City, Mexico, 63Department of Physics, University of Oxford, Oxford, United Kingdom, 64Department of Psychiatry, University of California San Diego, San Diego, CA, United States, 65Department of Psychology, Bangor University, Bangor, United Kingdom, 66Douglas Mental Health University Institute and Department of Psychiatry, McGill University, Montreal, QC, Canada, 67Department of Diagnostic Physics, Division of Radiology and Nuclear Medicine, Oslo University Hospital, Oslo, Norway, 68LIM44, Instituto e Departamento de Radiologia, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, Brazil, 69Department of Imaging & Pathology, Department of Radiology, University Hospitals Leuven, KU Leuven, Leuven, Belgium, 70Functional MRI Laboratory, University of Michigan, Ann Arbor, MI, United States, 71Institute of Neuroradiology, Goethe-University Frankfurt, Frankfurt, Germany, 72Center for Cognitive Aging and Memory, McKnight Brain Institute, Department of Clinical and Health Psychology, College of Public Health and Health Professions, University of Florida, Gainesville, FL, United States, 73Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, United States, 74Forensic & Neurodevelopmental Sciences, King's College London, London, United Kingdom, 75NeuRA Imaging, Neuroscience Research Australia, Randwick, Australia, 76Laboratory of Experimental Psychiatry, Instituto Nacional de Neurología y Neurocirugía, Mexico City, Mexico, 77Department of Psychological and Brain Sciences, Dartmouth College, Hanover, NH, United States, 78Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 79McKnight Brain Institute, AMRIS, University of Florida, Gainesville, FL, United States, 80Center for MR Research, University Children's Hospital, Zurich, Switzerland, 81Center of Functionally Integrative Neuroscience, Aarhus University Hospital, Aarhus, Denmark, 82Medical Physics, Mater Dei Hospital, Imsida, Malta, 83Department of Radiology and Nuclear Medicine, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Netherlands, 84504, Emanuel Institute of Biochemical Physics of the Russian Academy of Sciences, Moscow, Russian Federation, 85Psychiatry, University of Colorado Anschutz Medical Campus, Aurora, CO, United States, 86Clinical Neuroscience, MRI Centre, Karolinska Institutet, Clinical Neuroscience, MRI Centre, Sweden, 87Lewis Center for Neuroimaging, University of Oregon, Eugene, OR, United States, 88Department of Neurobiology and Behavior, Facility for Imaging and Brain Research (FIBRE) & Campus Center for Neuroimaging (CCNI), University of California, Irvine, Irvine, CA, United States, 89Spinoza Centre for Neuroimaging, Royal Netherlands Academy of Arts and Sciences, Amsterdam, Netherlands, 90Institute of Clinical Neuroscience and Medical Psychology, University Dusseldorf, Medical Faculty, Düsseldorf, Germany, 91Otorhinolaryngology, Head and Neck Surgery, University of Groningen, University Medical Center Groningen, Groningen, Netherlands, 92Brain Health Imaging Centre, Centre for Addiction and Mental Health, Toronto, ON, Canada, 93Melbourne Dementia Research Centre, Florey Institute of Neurosciences and Mental Health, Melbourne, Australia, 94Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia, 95Department of Radiology, University of Alabama at Birmingham, Birmingham, AL, United States, 96Centre for Human Brain Health, University of Birmingham, Birmingham, United Kingdom, 97Department of Diagnostic and Interventional Radiology, University Dusseldorf, Medical Faculty, Düsseldorf, Germany, 98Center for Cognitive Aging and Memory, McKnight Brain Institute, Department of Clinical and Health Psychology, College of Public Health and Health Professions. Department of Neuroscience, College of Medicine, University of Florida, Gainesville, FL, United States, 99Department of Radiology, TMU-Shuang Ho Hospital, New Taipei City, Taiwan, 100Department of Radiology and Psychiatry, Baylor College of Medicine, Houston, TX, United States, 101Perinatal Imaging & Health, King's College London, London, United Kingdom, 102Bioimaging Research Center, University of Georgia, Athens, GA, United States
This study measured field drift in eighty-seven 3T scanners using the water signal acquired by MRS. Frequency traces were plotted for each dynamic and severity of drift quantified. This dataset will be publicly available for benchmark and further analyses.
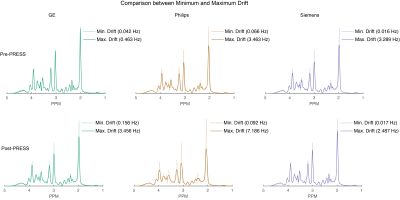
Figure 4. Comparison of simulated spectra with frequency drift applied between minimum and maximum drift for pre- and post-fMRI PRESS data. The minimum-drift case for each vendor (50% opacity) is overlaid with the maximum-drift case (opaque).

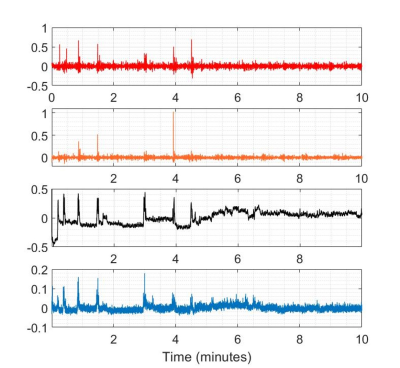
Figure 1. Individual transients of pre- and post-fMRI PRESS (plotted in blue and red, respectively) from the highest-drifting scanner. The frequency offset derived from modeling the water signals is plotted (middle). 360 averages correspond to 30 minutes total scan duration.