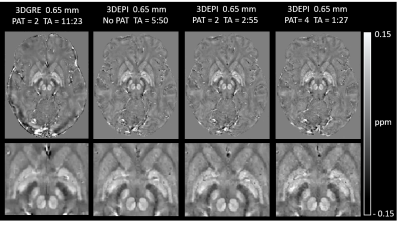
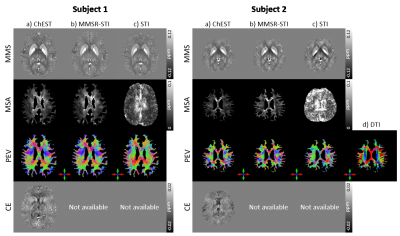
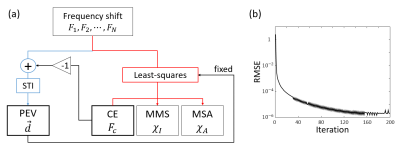
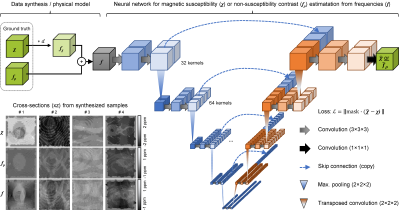
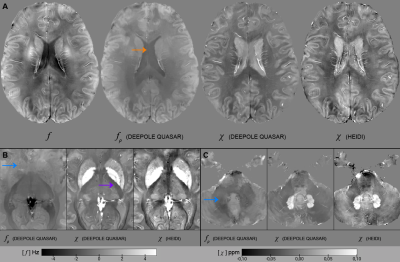
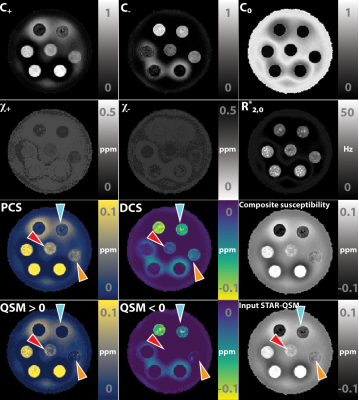
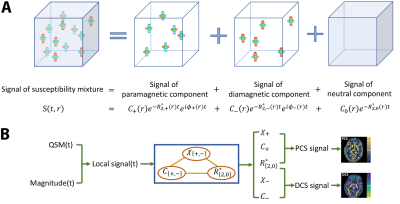
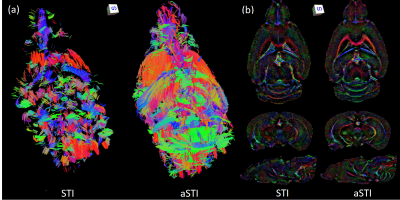
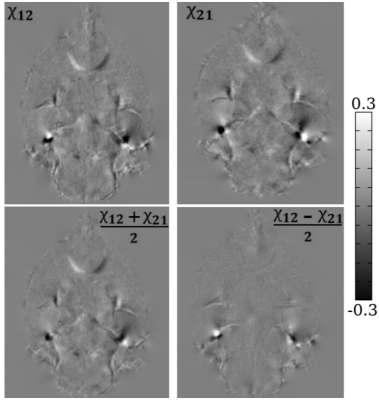
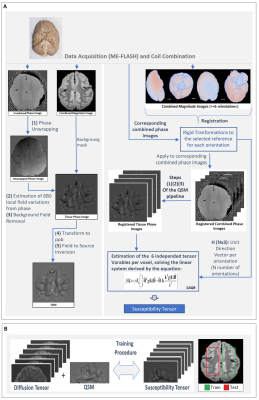
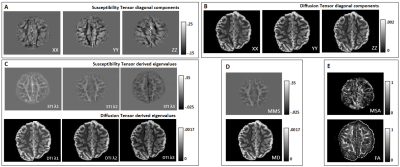
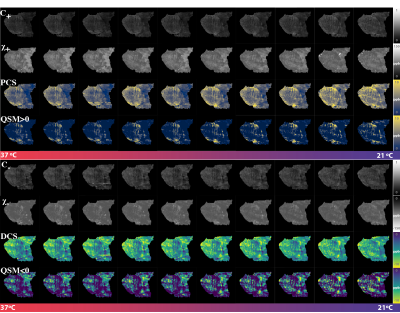
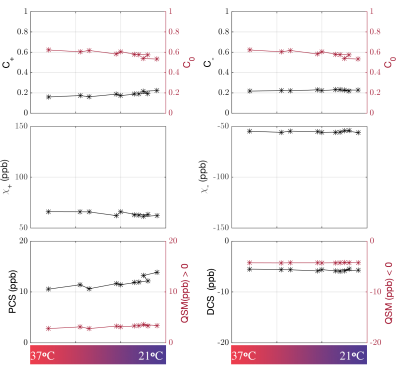
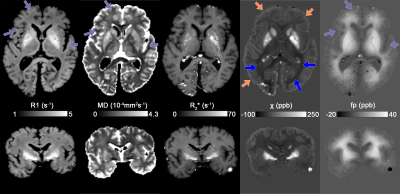
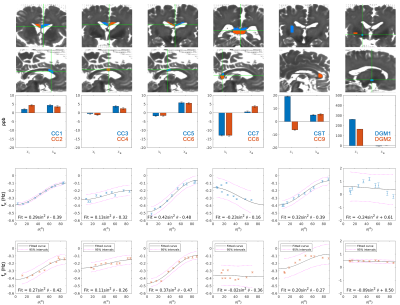
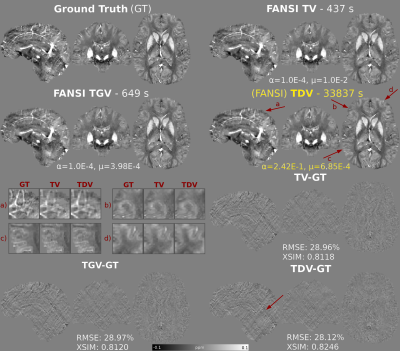
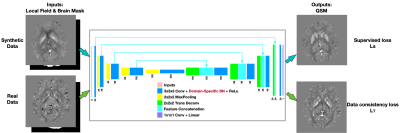
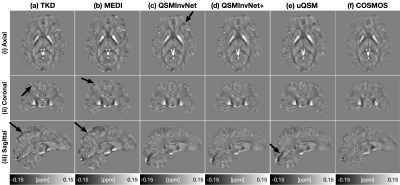
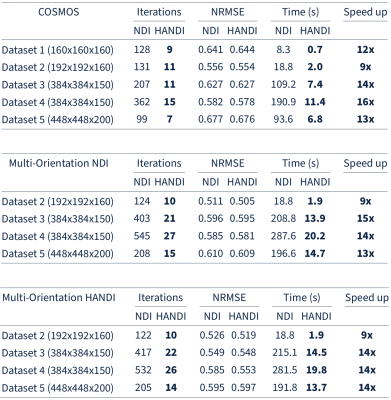
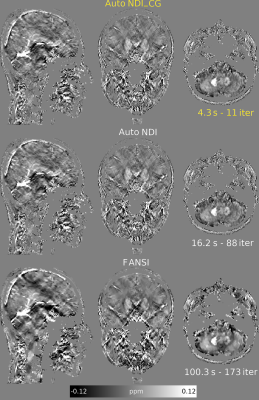
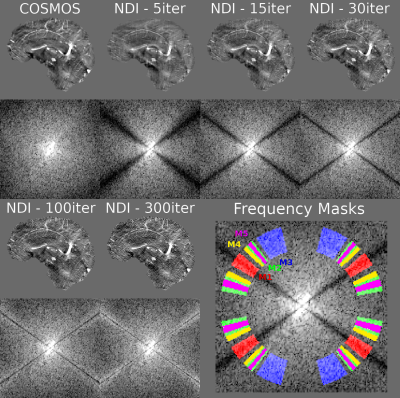
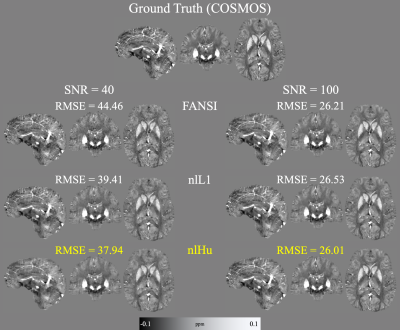
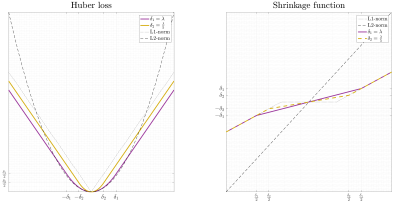
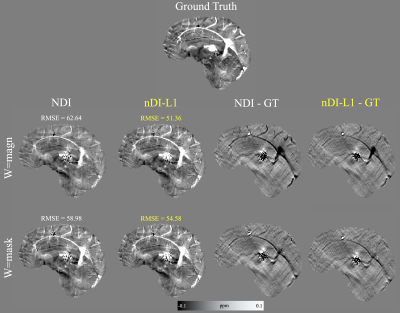
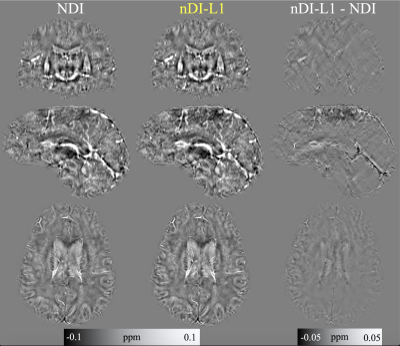
-
Quantitative susceptibility mapping MRI of brain iron and PET of β-amyloid predict cognitive decline during aging
Lin Chen1,2, Anja Soldan3, Kenichi Oishi1, Andreia Faria1, Marilyn Albert3, Peter van Zijl1,2, and Xu Li1,2
1Department of Radiology and Radiological Sciences, Johns Hopkins University, Baltimore, MD, United States, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3Department of Neurology, Johns Hopkins University, Baltimore, MD, United States
Greater volume of multiple brain regions was strongly associated with the rate of cognitive decline. Associations between brain iron and β-amyloid and longitudinal cognitive decline were weaker, with brain iron in the basal ganglia and entorhinal cortex predicting global decline.
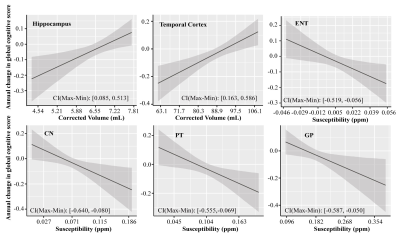
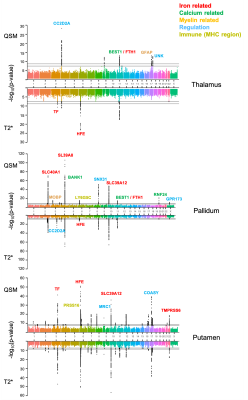
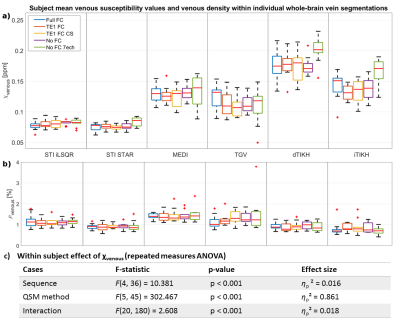
Figure 1 Associations of the corrected volume in hippocampus and temporal cortex, QSM in ENT, CN, PT and GP, to the annual change in global cognitive functions in all participants (n = 150). Annual change of cognitive function was calculated for each participant based on his or her trajectory of decline in the follow-up years after baseline MRI (up to 5 years), and it was plotted against baseline predictor, i.e. corrected volume or QSM. CI (Max-Min) = confidence interval of the difference between the annual change in global cognitive score at the minimum and maximum values of the predictor.
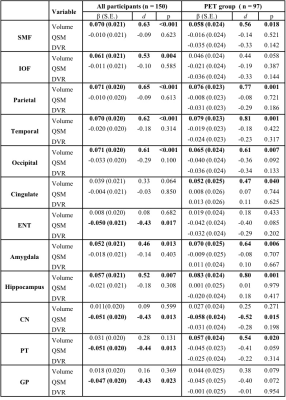
Table 1 Associations of structure volume, tissue iron load (QSM) and β-amyloid load (PET DVR) with rate of change in global cognitive composite scores (variable×time interaction in mixed-effects models) in all participants and participants in the PET group. d: effect size.
-
Prediction of Amyloid-β Deposition Using Multiple Regression Analysis of Quantitative Susceptibility Mapping
Ryota Sato1, Kohsuke Kudo2, Niki Udo3, Masaaki Matsushima4, Ichiro Yabe4, Akinori Yamaguchi2, Makoto Sasaki5, Masafumi Harada6, Noriyuki Matsukawa7, Tomoki Amemiya1, Yasuo Kawata1, Yoshitaka Bito1, Hisaaki Ochi1, and Toru Shirai1
1Healthcare Business Unit, Hitachi, Ltd., Tokyo, Japan, 2Department of Diagnostic Imaging, Hokkaido University Graduate School of Medicine, Hokkaido, Japan, 3Department of Psychiatry, Hokkaido University Graduate School of Medicine, Hokkaido, Japan, 4Department of Neurology, Faculty of Medicine and Graduate School of Medicine, Hokkaido University, Hokkaido, Japan, 5Institute for Biomedical Sciences, Iwate Medical University, Iwate, Japan, 6Department of Radiology, Tokushima University, Tokushima, Japan, 7Department of Neurology, Nagoya City University, Aichi, Japan
A prediction
model of Aβ deposition was created based on multiple regression analysis of
quantitative susceptibility mapping (QSM). The results showed that the model
could predict Aβ positivity at moderate accuracy.
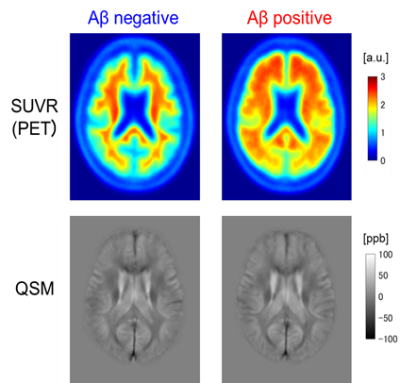
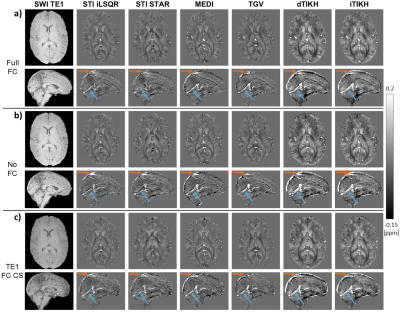
Figure 1. Mean images of SUVR and QSM for Aβ positive and negative patients.
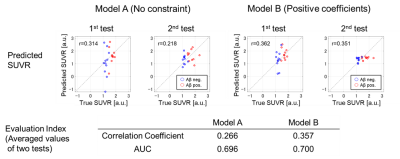
Figure 2. Evaluation results.
-
Quantifying iron deposition in Multiple System Atrophy via multi-echo Quantitative Susceptibility Mapping
Marta Lancione1,2, Matteo Cencini2,3, Mauro Costagli3,4, Graziella Donatelli2,5, Michela Tosetti2,3, Claudio Pacchetti6, Pietro Cortelli7,8, and Mirco Cosottini5
1IMT School for Advanced Studies Lucca, Lucca, Italy, 2IMAGO7 Foundation, Pisa, Italy, 3IRCCS Stella Maris, Pisa, Italy, 4Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Sciences (DINOGMI), University of Genova, Genova, Italy, 5Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy, 6Parkinson and Movement Disorder Unit, IRCCS Mondino Foundation, Pavia, Italy, 7Department of Biomedical and NeuroMotor Sciences, University of Bologna, Bologna, Italy, 8Clinica Neurologica, IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy
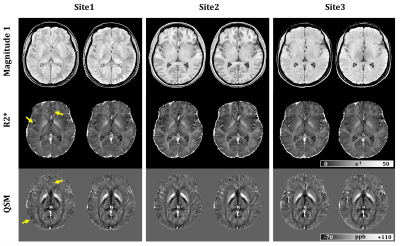
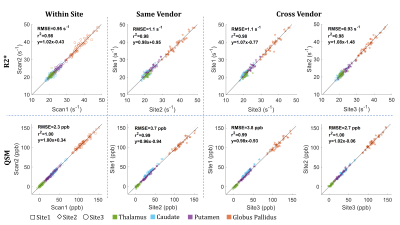
QSM can
detect the increase of iron deposition in healthy controls and MSA patients of
both parkinsonian and cerebellar variants. Short TEs enhances its diagnostic
performances unveiling previously unreported alteration of deep gray matter
nuclei.
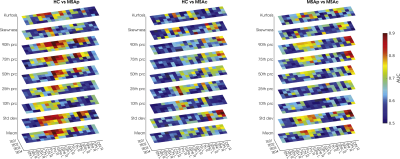
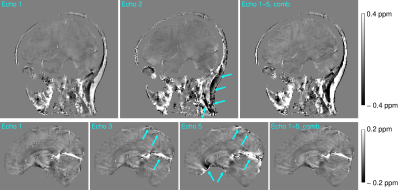
Figure 4: In
each plot the diagnostic accuracy for each group pair (HC vs MSA-p, HC vs MSA-c
and MSAp vs MSAc) is reported. The colors represent the values of the area
under the ROC curve (AUC) for each TE, for each ROI and for each histogram
feature. It can be noticed that AUC values are higher for short TEs.
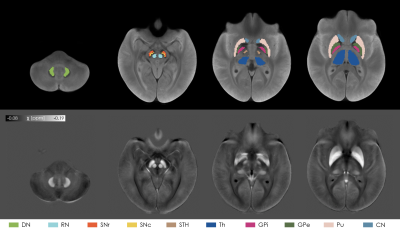
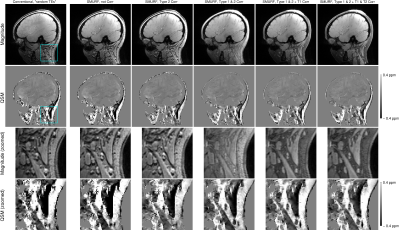
Figure 1:
The top row shows the ROIs used in the analysis overlaid onto the
study-specific template, which was computed from the across-TEs average of the
T2*-weighted image of each subject. In the bottom row the susceptibility maps computed
from the first TE averaged over all the subjects is displayed.
-
Clinical correlations of iron-rich deep grey matter of MS patients
Ibrahim Khormi1,2, Oun Al-iedani1,2, Amir Fazlollahi2,3, Bryan Paton2,4, Jeannette Lechner-Scott2,4,5, Abdulaziz Alshehri1,2, Kieran O'Brien6,7, Steffen Bollmann8, Rishma Vidyasagar9, Scott Ayton9, Anne-Louise Ponsonby9,10, and Saadallah Ramadan1,2
1School of Health Sciences, University of Newcastle, Newcastle, Australia, 2Hunter Medical Research Institute, Newcastle, Australia, 3CSIRO Health and Biosecurity, Brisbane, Australia, 4University of Newcastle, Newcastle, Australia, 5John Hunter Hospital, Newcastle, Australia, 6Siemens Healthcare Pty Ltd, Brisbane, Austria, 7ARC Training Centre for Innovation in Biomedical Imaging Technology, The University of Queensland, Brisbane, Australia, 8The University of Queensland, Brisbane, Australia, 9The Florey Institute of Neuroscience & Mental Health, Parkville, Australia, 10Murdoch Children's Research Institute, Royal Children's Hospital, University of Melbourne, Melbourne, Australia
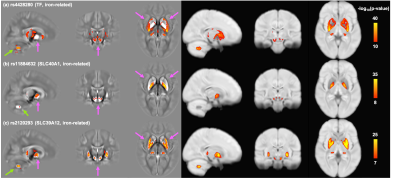
QSM metrics in caudate showed strong correlations with depression scores,
while pallidum and thalamus correlated significantly with anxiety.
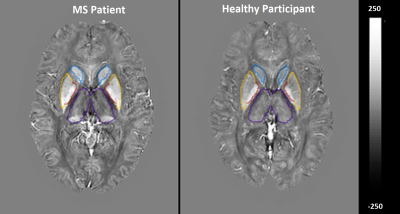
Figure 1. Quantitative
susceptibility maps within thalamus (purple), caudate (blue), pallidum (red)
and putamen (yellow) of a 38 years-old female RRMS vs. 40years-old female HCs.
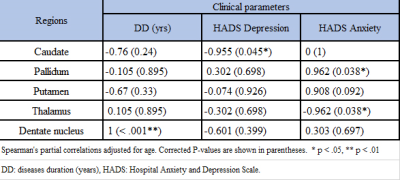
Table 3. Spearman’s correlation between iron-rich regions in deep grey matter
in MS cohort only.
-
Separation of positive and negative susceptibility contrast at 7 Tesla allows for a more detailed characterization of multiple sclerosis lesions
Julian Emmerich1,2, Frederik L. Sandig3, and Sina Straub1
1Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Faculty of Physics and Astronomy, University of Heidelberg, Heidelberg, Germany, 3Division Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany
It is
shown that the occurrence of bright multiple sclerosis lesions and have
different origins that can either be separately observed in positive or
negative susceptibility maps.
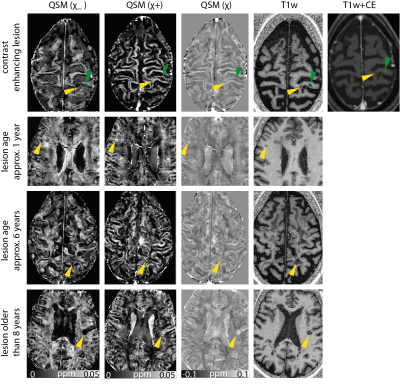
Figure 2:
Positive,
negative and conventional susceptibility maps, as well as a T1-weighted image
for lesions from three different patients. For the enhancing lesion, additionally
a contrast-enhanced T1-weighted image is shown. Lesion age is indicated on the
left and lesions are ordered according to their age. Green indicates a contrast
enhancing lesion, yellow arrows point to non-enhancing lesions.
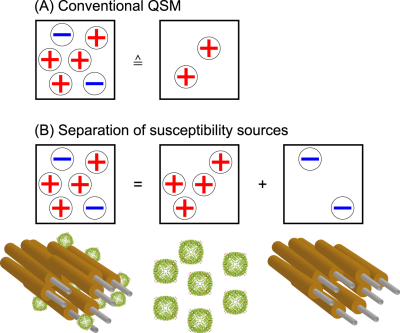
Figure1: The cancellation of the susceptibility effects within the same voxel in
QSM are illustrated.
-
Quantitative susceptibility mapping in the infant brain diagnosed with congenital heart disease
Zungho Zun1,2,3,4, Kushal Kapse1, Nicole Andersen1, Scott Barnett1,2,3,4, Anushree Kapse1, Kristina Espinosa1, Jessica Quistorff1, Catherine Lopez1, Jonathan Murnick1,3,4, Mary T. Donofrio2,3,5, and Catherine Limperopoulos1,2,3,4
1Division of Diagnostic Imaging and Radiology, Children's National Hospital, Washington, DC, United States, 2Division of Fetal and Transitional Medicine, Children's National Hospital, Washington, DC, United States, 3Department of Pediatrics, George Washington University, Washington, DC, United States, 4Department of Radiology, George Washington University, Washington, DC, United States, 5Division of Cardiology, Children’s National Hospital, Washington, DC, United States
Magnetic susceptibilities measured in the first two months of life were lower in the white matter and temporal lobe in infants diagnosed with congenital heart disease, and Bayley language scores evaluated at 18 months were associated with magnetic susceptibilities of the temporal lobe.
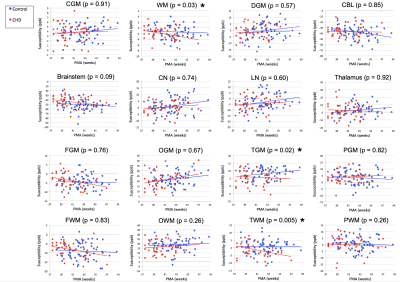
Figure 1. Regional magnetic susceptibilities measured in the brain of healthy control infants and those diagnosed with CHD in the first two months of life. Asterisks denote significant differences between control and CHD infants when controlling for PMA and accounting for repeated measures of the same infant.
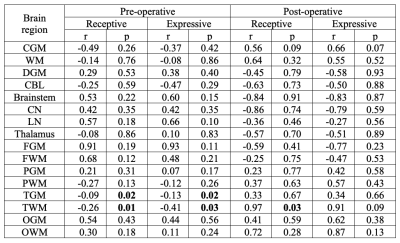
Table 2. Associations between magnetic susceptibilities measured in pre/post-operative MRI and Bayley-III receptive/expressive language scores. P values in bold indicate significant associations.
-
Quantitative Susceptibility Mapping of Venous Vessels in Neonates With Perinatal Asphyxia
Alexander Mark Weber1, Yuting Zhang2, Christian Kames3, and Alexander Rauscher1
1Pediatrics, UBC, Vancouver, BC, Canada, 2Radiology, Children’s Hospital of Chongqing Medical University, Chongqing, China, 3Physics, UBC, Vancouver, BC, Canada
QSM derived oxygen saturation values in healthy term and asphixia injured preterm and term neonates agrees well with past literature. CSVO2 in preterm and term neonates with HIE, however, were not found to be significantly different from each other or healthy controls.
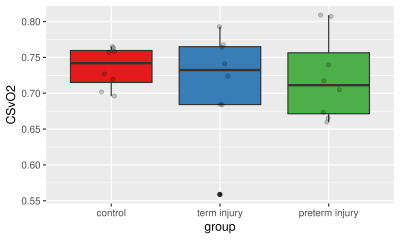
Figure 2. Boxplot of CSVO2 percentages by group. Grey circles are the ROI measurements from each subject.
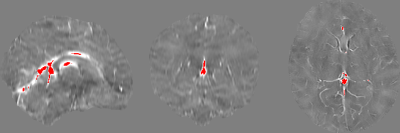
Figure 1. Sample internal veins selected after thresholding out the lower 99.75% χ values. As can be seen in these sagittal, coronal, and axial views from a sample healthy term neonate, the major veins that were left over include the straight sinus, inferior sagittal sinus and the internal cerebral vein. Note the weak contrast between grey and white matter and the basal ganglia due to the low myelin and iron content of the newborn brain.
-
Investigating the Effect of Positive Airways Pressure on Venous Oxygenation in Sickle Cell Anemia with Quantitative Susceptibility Mapping
Russell Murdoch1, Hanne Stotesbury2, Jamie Kawadler2, Dawn Saunders2, Fenella Kirkham2, and Karin Shmueli1
1Department of Medical Physics and Biomedical Engineering, University College London, London, United Kingdom, 2Imaging and Biophysics, Developmental Neurosciences, UCL Great Ormond Street Institute of Child Health, London, United Kingdom
Quantitative Susceptibility Mapping showed decreased venous oxygenation (Yv) in sickle cell anaemia (SCA), with significantly lower Yv in SCA subjects with silent cerebral infarcts vs without. APAP had no significant effect on Yv in SCA but treatment compliance correlated with Yv increases.
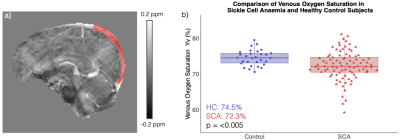
a) Sagittal slice from the QSM in a representative sickle cell anaemia (SCA) subject with the superior sagittal sinus (SSS) region of interest overlaid in red. b) Comparison of QSM venous oxygenation (Yv) in the SSS in subjects with sickle cell anaemia (SCA) and healthy controls (HC). Significantly lower Yv was observed in the SCA group which also showed a wider range of Yv values than the HC group.
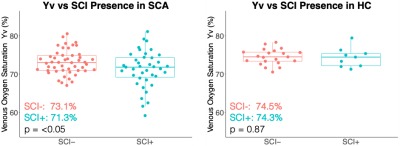
Comparison of QSM venous oxygenation (Yv) measured in the superior sagittal sinus of subjects with sickle cell anaemia (SCA) and healthy controls (HC) with (SCI+) and without (SCI-) silent cerebral infarcts (SCI). In SCA, significantly lower Yv was measured in subjects with SCI relative to those without. No significant differences in Yv were observed between the SCI+ and SCI- groups in HC subjects.
-
Validation Study of Venous Oxygenation in Internal Cerebral Vein in Patients with Sickle Cell Disease by QSM and CISSCO Method
Jian Shen1, Aart Nederveen2, and John Wood1,3
1Biomedical Engineering, University of Southern California, Los Angeles, CA, United States, 2Radiology, Academic Medical Center, Amsterdam, Netherlands, 3Children's Hospital Los Angeles, Los Angeles, CA, United States
Both QSM and CISSCO methods reveal the group differences in oxygen saturation in the internal cerebral vein, indicating the existence of "physiological shunting".
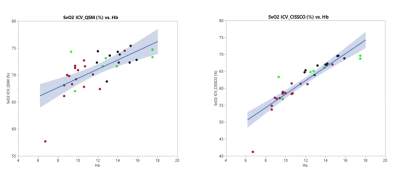
Figure 3.
Relationship between venous oxygen saturation and hemoglobin measured by QSM and CISSCO.
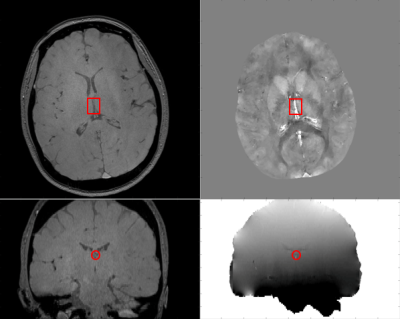
Figure 1.
Acquired image and the internel cerebral vein. Magnitude in axial view (top left), magnitude in
coronal view(bottom left), phase in coronal view (bottom right) and processed
susceptibility map (top right).
-
Investigating the Magnetic Susceptibility of Silent Cerebral Infarcts in Sickle Cell Anaemia Using Two Different Gradient Echo Acquisitions
Russell Murdoch1, Hanne Stotesbury2, Jamie Kawadler2, Dawn Saunders2, Fenella Kirkham2, and Karin Shmueli1
1Department of Medical Physics and Biomedical Engineering, University College London, London, United Kingdom, 2Imaging and Biophysics, Developmental Neurosciences, UCL Great Ormond Street Institute of Child Health, London, United Kingdom
Silent cerebral infarcts in the parietal lobes of the brain had significantly lower magnetic susceptibility than frontal lobe lesions. Strong correlations were observed between lesion susceptibilities measured using two different GRE acquisitions suggesting χ referencing is not needed.
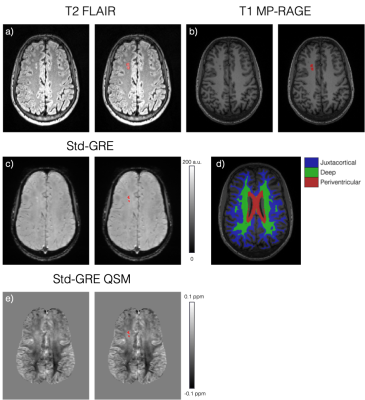
a) Axial slice of a T2-weighted FLAIR image showing two silent cerebral infarcts (SCI) in a representative sickle cell anaemia subject (right: SCI ROI overlaid in red). b) Axial slice of the T1-weighted MP-RAGE image showing the same lesions appearing hypointense. c) Similar axial slice of the Std-GRE magnitude at TE = 27ms in the same subject. d) A different axial slice from the MP-RAGE showing the segmented juxtacortical, deep and periventricular white matter regions. e) QSM in the same axial slice as in c) showing a slight χ difference between the lesions and normal appearing WM.
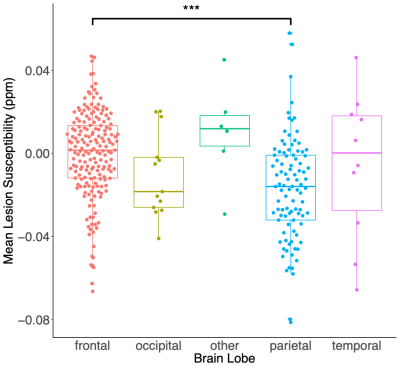
Mean SCI lesion χ within each brain lobe in the combined sickle cell anaemia and healthy control groups. Mean SCI χ are significantly lower for lesions in the parietal lobe relative to the frontal lobe (diff = -0.017ppm, p<0.001). In white matter, myelin is the key diamagnetic (negative) susceptibility source. Therefore, these results suggest reduced myelin concentrations within lesions in the frontal lobe relative to the parietal lobe. No significant χ differences were observed between SCI any other regions.
-
Improved Regularization for Quantitative Susceptibility Mapping of Liver Iron Overload
Julia V Velikina1, Ruiyang Zhao1,2, Collin Buelo2, Alexey A Samsonov1, Scott Reeder1,2,3,4,5, and Diego Hernando1,2
1Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 3Biomedical Engineering, University of Wisconsin - Madison, Madison, WI, United States, 4Emergency Medicine, University of Wisconsin - Madison, Madison, WI, United States, 5Medicine, University of Wisconsin - Madison, Madison, WI, United States
We optimize the use of additional information provided by multi-echo imaging to regularize the QSM inversion problem. This approach resulted in significantly reduced shading artifact, improved quality of susceptibility maps, and higher repeatability of measurements.
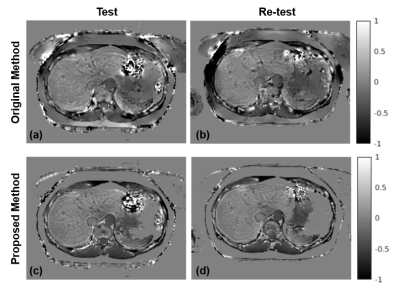
Figure 5. Compared to the original liver QSM method (a: test, b: re-test), the proposed method (c: test, d: re-test) produces more consistent (repeatable) susceptibility maps. The improved repeatability may be explained by the fact that the proposed method is less sensitive to errors in the field map induced by air/tissue interface due to adaptive data weighting, which, together with fat constraint, reduces shading artifacts.
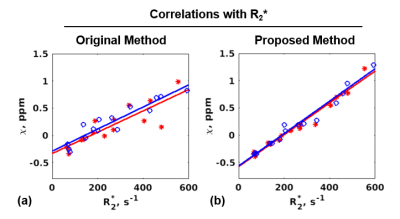
Figure 3. Correlation of R2* values in the liver with susceptibility values and the corresponding linear regression lines for test (red) and re-test (blue) exams obtained with (a) the original method (slopes 0.002/0.002, y-intercepts -0.334/-0.289, R2=0.858/0.939); (b) the proposed method (slopes 0.0029/0.003, y-intercepts -0.577/-0.566, R2=0.982/0.985). These slopes/intercepts are consistent with previously reported5 correlation results (slope 0.0028, y-intercept -0.54).
-
Quantitative Susceptibility Mapping of Liver Iron Overload using Deep Learning
Ruiyang Zhao1,2, Collin J Buelo2, Julia V Velikina1, Steffen Bollmann3, Ante Zhu4, Scott B Reeder1,2,5,6,7, and Diego Hernando1,2
1Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 3School of Information Technology and Electrical Engineering, University of Queensland, Brisbane, Australia, 4GE Global Research, Niskayuna, NY, United States, 5Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 6Medicine, University of Wisconsin-Madison, Madison, WI, United States, 7Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States
A novel deep learning-based QSM method was developed and evaluated for the quantification of liver iron overload. Validation results demonstrated promising performance and agreement with reference susceptibility values across a wide range of iron overload.
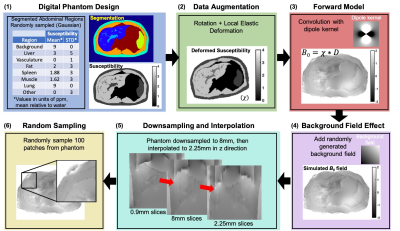
Figure 1: Training of DL QSM: (1) Digital torso 3D phantom with various random-susceptibility regions; (2) Augmented with rotation and local elastic deformation; (3) Convolved with a dipole kernel (voxel:1.56x1.56x0.9mm3, matrix:2563) to create a field map; (4) A random background field was added; (5) The field map was down-sampled to 8mm slices (in-vivo resolution), then interpolated to 2.25mm to enable a deeper network; (6) 643 patches (n=100) were randomly pulled for training.
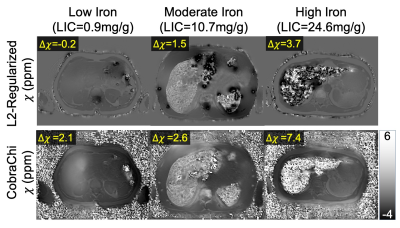
Figure 2: Representative
susceptibility maps from a previously proposed L2-regularized liver QSM method
(top), and the proposed CobraChi DL-based method (bottom), in patients with
various levels of liver iron. Compared to the L2-regularized method, CobraChi
may provide better robustness at high iron levels, although some artifactual
shading remains at low iron levels. For both QSM methods, Δχ
of the liver is measured relative to subcutaneous fat.
-
Comparison of True Susceptibility Weighted Imaging (tSWI) with SWI and QSM for Intracranial Hemorrhage
Ashmita De1, Derek J. Emery2, Kenneth S. Butcher3, and Alan H. Wilman1
1Department of Biomedical Engineering, University of Alberta, Edmonton, AB, Canada, 2Department of Radiology and Diagnostic Imaging, University of Alberta, Edmonton, AB, Canada, 3Division of Neurology, Department of Medicine, University of Alberta, Edmonton, AB, Canada
tSWI removes the phase artifacts that are observed in SWI, provides
better susceptibility weighting than magnitude and improves contrast visualization
within the hemorrhage as compared to QSM. tSWI can be used for hemorrhage
visualization when SWI cannot provide a clear depiction.
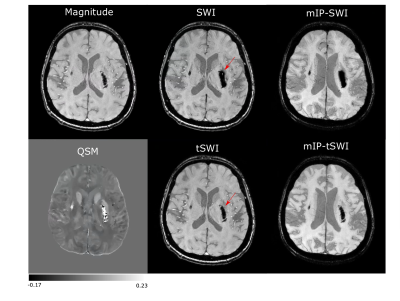
Figure 1: Magnitude, SWI,
tSWI, QSM and mIP from SWI and tSWI in a patient with an acute Day 2 hemorrhage. It demonstrates the blooming effect in SWI of hemorrhage.
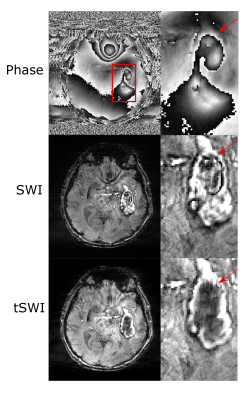
Figure 2: A comparison between phase, SWI and tSWI in a patient with Day 12 hemorrhage to show phase wrap artifacts are present in SWI for large hemorrhage which gets removed in tSWI as shown by the red arrow.
-
Radiomic Features on Quantitative Susceptibility Mapping Classify Amyotrophic Lateral Sclerosis Patients from Mimics
Anja Samardzija1, Thanh Nguyen2, Elizabeth Sweeney2, Kailyn Lee2, Ilhami Kovanlikaya2, Yi Wang 2, Andrew Schweitzer2, and Apostolos Tsiouris2
1Electrical and Computer Engineering, Cornell University, Highlands, NJ, United States, 2Weill Cornell Medicine, New York City, NY, United States
QSM is used to classify ALS patients from similar clinical presentations. A random forest classification model is applied on the radiomic features of the primary motor cortex which has accuracy of 0.8, specificity of 0.75, and sensitivity of 0.84.
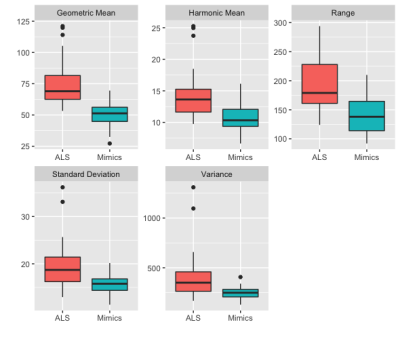
Figure 1. Distributions of the most important variables of the random forest by diagnosis group.
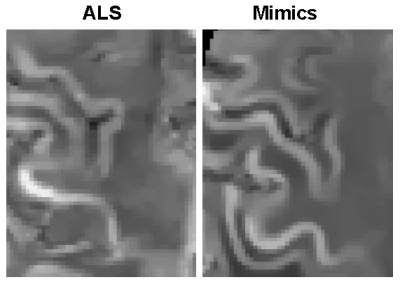
Figure 2. Example of QSM images of the primary motor cortex region obtained from ALS and mimics patients.
-
Quantitative susceptibility mapping in the basal ganglia of systemic lupus erythematosus patients with neuropsychiatric complaints
Marjolein Bulk1, Thijs van Harten1, Boyd Kenkhuis1, Francesca Inglese1, Ingrid Hegeman1, Sjoerd van Duinen1, Ece Ercan1, Cesar Magro-Checa1,2, Jelle Goeman1, Christian Mawrin3, Mark van Buchem1, Gerda Steup-Beekman1, Tom Huizinga1, Louise van der Weerd1, and Itamar Ronen1
1Leiden University Medical Center, Leiden, Netherlands, 2Zuyderland Medical Center, Heerlen, Netherlands, 3Otto-von-Guericke University, Magdeburg, Germany
No significant
differences in local susceptibility in the basal ganglia were found between SLE
patients with NP complaints and healthy controls, suggesting that in NPSLE, neuroinflammation
is not necessarily associated with iron accumulation.
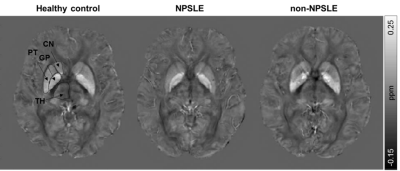
Figure 2. Representative axial QSM images of a
healthy control, NPSLE patient and non-NPSLE patient. The regions of interest are
indicated in the healthy control. TH = Thalamus; CN = Caudate nucleus; PT =
Putamen; GP = Globus pallidus.
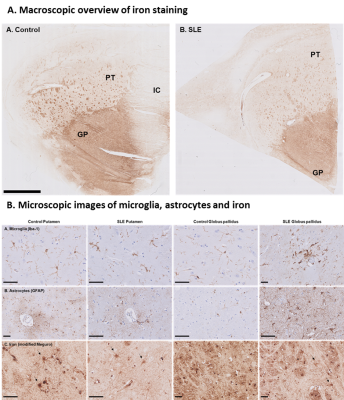
Figure 5. Iron in control and SLE brain. Globus pallidus showed higher
staining intensity compared to putamen. Within the putamen small areas of increased
iron were found originating from myelinated fiber bundles. Iron was
predominantly found in oligodendrocytes and myelin, to a lesser extent, in
neurons, microglia and astrocytes (arrows) in both control and SLE.
-
Scan Efficiency Optimisation for Quantitative Susceptibility Mapping of White Matter at 7T.
Jan Sedlacik1,2, Raphael Tomi-Tricot1,3, Pip Bridgen1,2, Tom Wilkinson1,2, Sharon Giles1,2, Karin Shmueli4, Jo V Hajnal1,2, and Shaihan J Malik1,2
1Centre for the Developing Brain, School of Biomedical Engineering & Imaging Sciences, King’s College London, London, United Kingdom, 2Biomedical Engineering Department, School of Biomedical Engineering & Imaging Sciences, King’s College London, London, United Kingdom, 3MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom, 4MRI Group, Department of Medical Physics and Biomedical Engineering, University College London, London, United Kingdom
Optimal scanning parameters were found and tested for quantitative
susceptibility mapping
at
7T aiming to most efficiently assess the white matter microstructure. The optimum was found for gradient echo imaging without RF spoiling, TR=29ms,
longest TE=26ms and FA=15.5°.
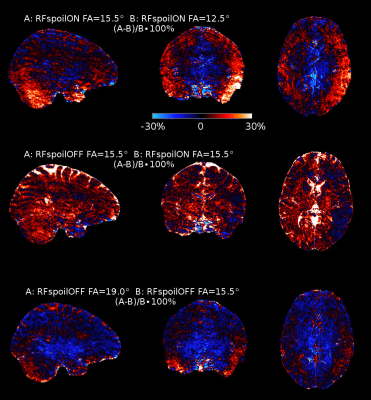
Figure 2:
Maps of the relative signal changes
(A-B)/B∙100%
between the different scan settings. The colour bar is scaled from -30
to 30%. Temporal and cerebellar regions benefit when increasing FA,
but signal is lost in the central brain region (top). Turning
the RF spoiling
OFF increased
the signal in the overall brain and particularly for the CSF (middle). Increasing the FA for RF spoiling
OFF, however, shows more signal loss in the overall brain (bottom).
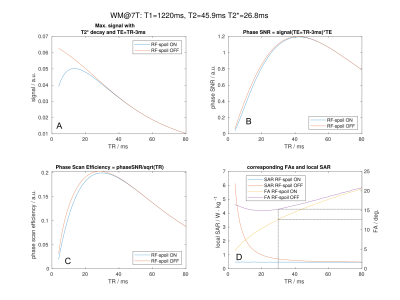
Figure 1: WM
signal
magnitude
(A)
for
different TE, TR and FA settings following
equations 1 and 2,
phase
SNR (B),
scanning efficiency (C)
and
the
corresponding FAs
and imposed SAR (D).
-
Whole brain CSF segmentation for consistent zero-referencing and longitudinal study applicability of MEDI+0
Alexey Dimov1, Thanh Nguyen1, Susan Gauthier2, and Yi Wang3
1Radiology, Weill Cornell Medicine, New York, NY, United States, 2Neurology, Weill Cornell Medicine, New York, NY, United States, 3Weill Cornell Medicine, New York, NY, United States
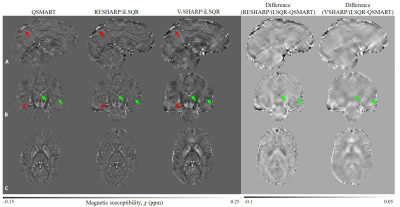
The role of CSF segmentation for zero-referenced MEDI+0 is demonstrated. In the present study we show that it is essential to enforce consistency in the CSF mask between different timepoints to ensure value reproducibility of the QSM
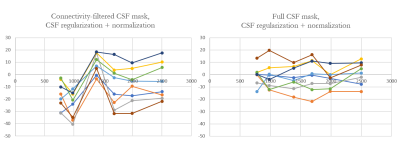
Figure 2. Comparison of the MS lesion susceptibility trajectories estimated using CSFvent mask (left) and the full CSFbrain (right) masks. Note synchronous jumps in
measured susceptibility values occurring with CSFvent
Figure 3. Comparison of the CSFvent for different datasets in longitudinal study
-
Artifact Evaluation of Quantitative Susceptibility Mapping Reconstructions Using Mask Parameter Perturbation
Priya S Balasubramanian1,2, Alexandra Grace Roberts1,2, Pascal Spincemaille2, Thanh Nguyen 2, and Yi Wang1,2
1Cornell University, New York City, NY, United States, 2Weill Cornell Medical College, New York City, NY, United States
A method of perturbing tissue mask is proposed to map artifacts associated with strong susceptibility sources recognized as a major cause of errors in QSM algorithms and is validated in a numerical phantom of a known susceptibility distribution and in vivo COSMOS reference data.
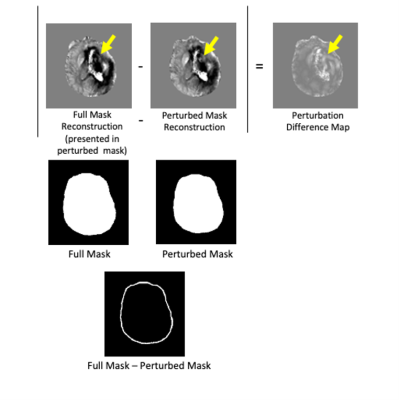
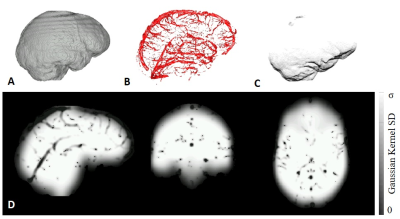
Figure 1. PDM is the absolute value of the difference between the full mask reconstruction, and the perturbed, 5mm eroded mask reconstruction. Artifacts shown with yellow arrows.
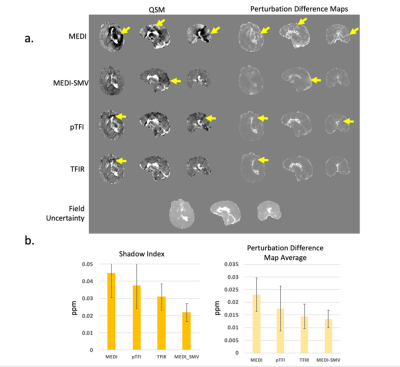
Figure 4 a) QSM and PDM in a hemorrhage case. Shown are MEDI, MEDI SMV, TFI, TFIR, and the field uncertainty. b) shadow score and PDM average for each method for 11 cases. Both scores decrease together as visual image artifacts decrease in a).
-
Regional Susceptibility Reconstruction Improves Artifact Incidence and Error in Quantitative Susceptibility Mapping through POSSUM
Priya S Balasubramanian1,2, Pascal Spincemaille2, Thanh Nguyen2, and Yi Wang1,2
1Cornell University, New York City, NY, United States, 2Weill Cornell Medical College, New York City, NY, United States
A quantitative susceptibility mapping method is proposed that combines regional mapping with total field inversion for improved error and artifact reduction.
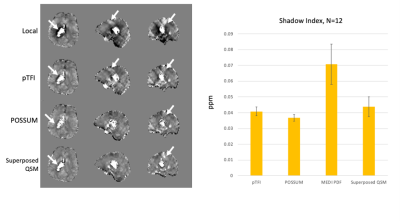
Figure 4– (left) Representative hemorrhage case with reconstructions, arrows show artifacts, (right) Shadow scores for 12 cases.
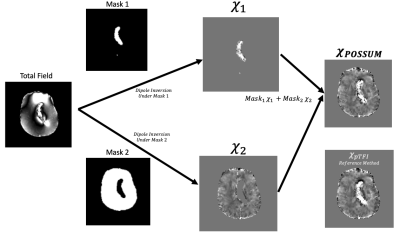
Figure 1 –Algorithmic flow chart for POSSUM
-
Quantitative Susceptibility Mapping in Preoperative Assessment of Microvascular Invasion of Hepatocellular Carcinoma: a Preliminary Study
Chang Liu1, Hongru Jia1, Weiqiang Dou2, Jing Ye1, and Xianfu Luo1
1Northern Jiangsu People’s Hospital, Yang zhou, China, 2GE Healthcare,MR Research China, Bei jing, China
QSM imaging can be considered a potential technique for noninvasive preoperational assessment of MVI in hepatocellular carcinoma.
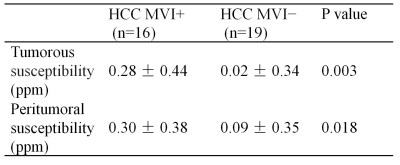
Table1. The susceptibility of tumorous and peritumoral from MVI+ and MVI− HCC.
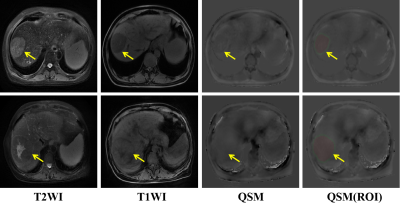
Figure1. Hepatectomy pathological confirmed hepatic carcinoma (Yellow arrow) with MVI− (Top row) and MVI+ (Bottom row). ROIs were placed on the maximal slice of tumor (red zone) and peritumor (green zone). The tumorous and peritumoral susceptibility of HCC with MVI− and MVI+ was -0.12 ppm, -0.09 ppm, 0.13 ppm, 0.16 ppm, respectively.