-
High-resolution, low-SAR 3D T2 relaxometry with COMBINE
Peter J Lally1, Matthew Grech-Sollars2,3, Joely Smith3,4, Ben Statton5, Paul M Matthews1,6, Karla L Miller7, and Neal K Bangerter4
1Department of Brain Sciences, Imperial College London, London, United Kingdom, 2Department of Surgery and Cancer, Imperial College London, London, United Kingdom, 3Department of Imaging, Imperial College Healthcare NHS Trust, London, United Kingdom, 4Department of Bioengineering, Imperial College London, London, United Kingdom, 5MRC London Institute of Medical Sciences, London, United Kingdom, 6UK Dementia Research Institute Centre at Imperial College, London, United Kingdom, 7Wellcome Centre for Integrative Neuroimaging, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom
Here we describe a super-resolution 3D T2
relaxometry approach using an unbalanced SSFP acquisition with very low flip
angle RF pulses (α≤1°), and apply this in a phantom. The proposed approach provides new options for high-resolution,
low-SAR T2 relaxometry experiments in a range of tissues.
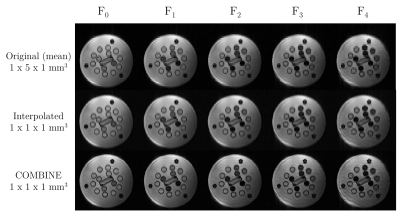
Figure 4: Top) Mean
low-resolution images (1x5x1mm3) for each of the different F-states
in the T2-COMBINE acquisition (TR=8ms, TE=4ms, α=1°).
Centre) Bicubic interpolation of the mean
low-resolution images (top) to a nominal voxel size of 1mm isotropic.
Bottom) Corresponding COMBINE reconstruction to a nominal voxel size of
1mm isotropic.
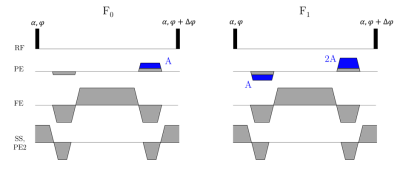
Figure 2: Schematic of the
pulse sequence for the T2-COMBINE experiment. Higher order F-states
are reached by adding integer increments of the unbalanced gradient area
(denoted A) either side of the readout.
-
Radial Fast Spin Echo MRI with Compressed Sensing for Simultaneous ADC and T2 Mapping
Lars Bielak1,2, Thomas Lottner1, and Michael Bock1,2
1Dept.of Radiology, Medical Physics, Medical Center University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 2German Cancer Consortium (DKTK), Partner Site Freiburg, Freiburg, Germany
A novel sequence design and reconstruction for simultaneous ADC and T2
mapping is presented. Simulations and phantom measurements show accurate measurement of diffusion ADC and T2 with as few
as 11 spokes per TE, and 45 different TEs.
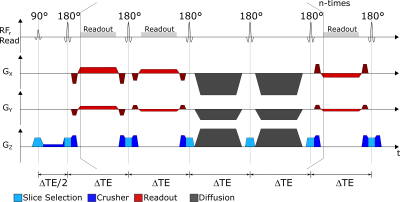
Sequence diagram of the proposed sequence. It employs
standard radial FSE readouts, interleaved with diffusion blocks. The diffusion
blocks can be placed after arbitrary numbers of readout blocks. The figure shows one diffusion block after 2 readout blocks each. For simulation and measurements, we use a combination of 3 readout blocks and one diffusion block (not shown).
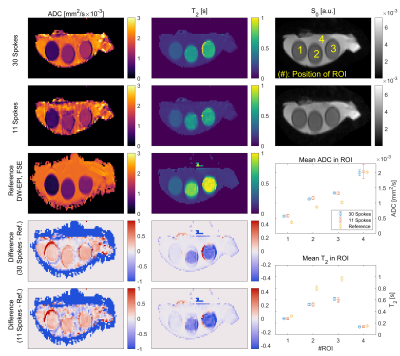
Results of the phantom measurement. The left column shows ADC
values, the middle column shows T2 values. The first two rows show the
reconstruction result for different number of spokes per ΔTE-b-pair
(30 and 11 spokes). The third row shows reference measurements using
conventional DW-EPI and FSE sequences. Rows 4 and 5 show corresponding
difference images. The two plots on the bottom right show a ROI based analysis
for all cases. The position of the ROI is indicated in the top right plot.
-
T2 Quantification in Brain Using Three Dimensional Fast Spin Echo Imaging with Long Echo Trains
Jeff Snyder1 and Alan H Wilman1
1Biomedical Engineering, University of Alberta, Edmonton, AB, Canada
A 3D FSE method using long echo trains is proposed for quantification of T2 in brain with mapping based on Bloch equation and Echo Phase Graph (EPG) simulations. Echo train lengths of 96 and 192 were investigated (total scan times of 8:12 and 2:38) at 3 T, with isotropic resolutions of 0.9 and 1.3 mm3.
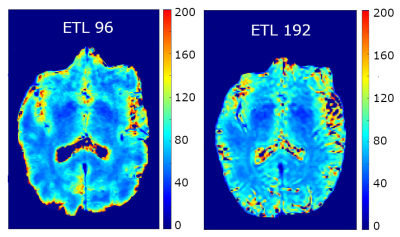
T2 maps for the ETL = 96 (left) and ETL = 192 (right)
variable flip angle trains computed from the two echo times of 6 and 132 ms. Noise is more visible in the 192 case. Note that the slice for the 192 case is slightly
offset from the 96 case due to the difference in resolution (0.9 mm3
vs 1.3 mm3)
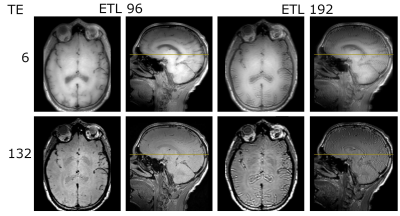
Images acquired using the proposed echo trains (left four
images – 96, and right four – 192). The
top row are all images at TE = 6 ms, and the bottom is at TE = 132 ms. Axial and sagittal views are shown for each
combination of TE and train length.
-
Improved echo-split GRASE imaging: a single-shot parametric T2 mapping protocol with removal of contamination from multiple echo pathways
Mei-Lan Chu1, Tzu-Cheng Chao2, Nan-kuei Chen3, and Hsiao-Wen Chung1
1Graduate Institute of Biomedical Electronics and Bioinformatics, National Taiwan University, Taipei, Taiwan, 2Mayo Clinic, Rochester, MN, United States, 3University of Arizona, Tucson, AZ, United States
Our novel single-shot T2 mapping framework removes signal contamination of high-order echoes and reliably generate accurate T2 relaxation times.
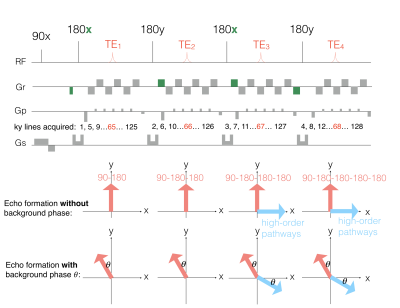
The single-shot ES-GRASE sequence. The areas and polarities of prewinding and rewinding gradients (i.e. the green gradient) of the readout gradient are designed to remove high-order echo pathways. The alternating direction of the refocusing RF pules (i.e. 180x-180y-180x-180y) can generate 90° phase difference between the primary and residual high-order echo pathways. The phase difference is 90+2𝜃° when the background phase is involved.
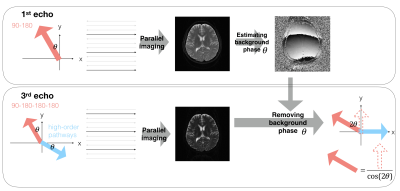
Data preprcessing frameowrk: First, images of four echoes were reconstructed using parallel imaging techniques, and the N/2 ghost was removed using phase cycling method. Second, the background phase was estimated from the first-echo image (see the upper panel). Third, rotating the signal from the third and fourth echo with the estimated background phase . The real part of the rotated signal is only from the signal of the primary echo pathway, with which the primary echo signal can be calculated by basic Trigonometry (see the lower panel in which only the third echo is shown as an example).
-
T2-Shuff-LL: Multi-contrast 3D Shuffling Combining Fast Spin-Echo and Look-Locker Gradient Echo
Jonathan Tamir1,2,3, Ken-Pin Hwang4, Naoyuki Takei5, and Suchandrima Banerjee6
1Electrical and Computer Engineering, The University of Texas at Austin, Austin, TX, United States, 2Diagnostic Medicine, Dell Medical School, The University of Texas at Austin, Austin, TX, United States, 3Oden Institute for Computational Engineering and Sciences, Austin, TX, United States, 4Imaging Physics, MD Anderson Cancer Center, Houston, TX, United States, 5MR Applications and Workflow, GE Healthcare, Hino, Tokyo, Japan, 6MR Applications and Workflow, GE Healthcare, Menlo Park, CA, United States
We propose a 3D shuffled multi-contrast acquisition combining multi-echo spin-echo and inversion-recovery spoiled gradient echo. As the acquisition is sensitive to T1 and T2, multi-contrast and quantitative images can be recovered from a single scan.
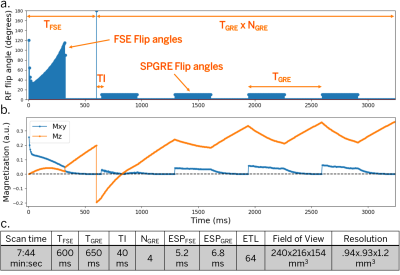
Figure 1. EPG Simulation and scan parameters. (a) RF flip angles (90-degree excitation not shown). (b) Transverse and longitudinal magnetization for T1=1600 ms, T2=80 ms (after arriving at a stable equilibrium magnetization). (c) Scan parameters for simulation and phantom experiments. The first acquisition block uses an FSE train with non-selective variable refocusing flip angles and a fast recovery tip-up pulse. After an adiabatic inversion pulse, a series of SPGRE blocks are played in sequence.

Figure 4. Animated gif of the reconstructed time series of images at three different slices in the ISMRM-NIST phantom, corresponding to the three plates of contrast spheres. In total, a time series of 300 images are reconstructed.
-
Clinical Evaluation of Isotropic MAVRIC-SL at 3T
Zoe Doyle1, Daehyun Yoon 1, Philip Lee1, Brian Hargreaves1, and Kathryn Stevens1
1Stanford University, Stanford, CA, United States
Our fast isotropic 3D MSI technique can facilitate the improved visualization of post-operative complications at 3T MRI by enabling the generation of clinically useful reformats in arbitrary imaging planes.
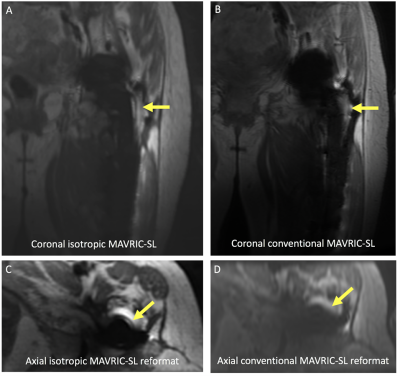
Fig 2. (A,B) Coronal and (C,D) axial reformatted images of a painful left revision total hip replacement in a 61-year-old female demonstrating abnormal signal adjacent to the lateral aspect of the femoral component on isotropic images (arrow). However, this abnormality cannot be seen on conventional MAVRIC SL images, despite the overall image quality being graded higher. Signal abnormality corresponded to fluid on inversion recovery sequences, confirming loosening (not shown).
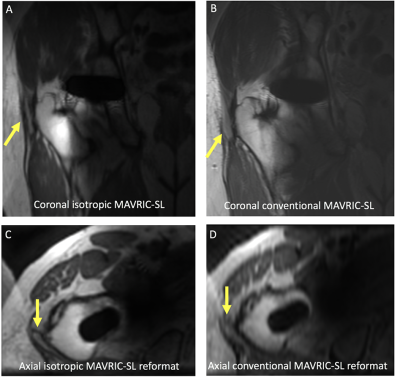
Fig 3. (A,B) Coronal and (C,D) axial reformatted images of a painful right total hip replacement in a 73-year-old male. A discrete trochanteric fluid collection is present (arrow), but is less well seen on conventional MAVRIC SL, particularly along the superior margin. Axial reformatted images confirm the presence of a trochanteric fluid collection, but the collection is less well seen on an axial reformatted conventional MAVRIC SL image (arrow).
-
Can we achieve a better performance in metamaterial-assisted MRI combined to an SLR-based fast spin-echo sequence?
Ekaterina A. Brui1, Stanislas Rapacchi2, David Bendahan2, and Anna Andreychenko1,3
1Department of Physics and Engineering, ITMO University, Saint Petersburg, Russian Federation, 2Aix-Marseille Universite, CNRS, Centre de Résonance Magnétique Biologique et Médicale, Marseille, France, 3Center of Diagnostics and Telemedicine Technologies, Department of Health Care of Moscow, Research and Practical Clinical, Moscow, Russian Federation
SLR-based FSE together with the metamaterial-based coil for wrist MRI allowed to increase the slice selectivity while still being within the safe SAR limits. The actual energy deposition was decreased as compared to a conventional RF setup.
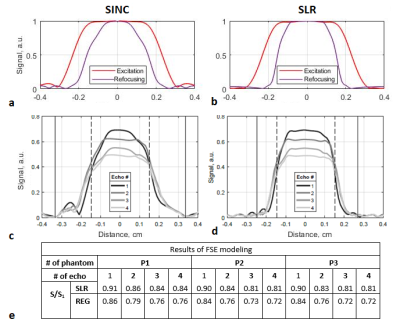
Excitation (red curves) and refocusing (purple curves) profiles of
regular pulses (a), which are used by default in 1.5 T Siemens Magnetom Avanto
scanner for FSE in "Low SAR" mode, and of the constructed SLR pulses (b) for a
sample with neglected relaxation. Refocusing profiles of four echo signals obtained in steady-state FSE
modeling with regular pulses (c) and SLR pulses (d) (T1 =
329 ms). Black dashed vertical lines indicate the desired slice thickness (TH),
black solid vertical lines indicate the entire refocused area of profiles (TH1). (c) Selectivity metric (S/S1) in FSE modeling.
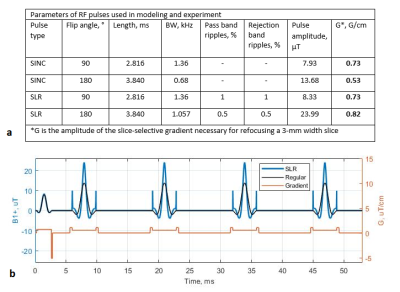
(a) Parameters
of RF pulses used in modeling and experiment. (b) The evolution in time of RF amplitudes (black and blue curves for regular and SLR-based FSE, respectively) and
slice selection gradient amplitudes with crushers (orange curve) that were generated. An additional crusher gradient was applied in the
end of the cycle (not shown), in order to fully spoil the transverse
magnetization. The
parameters of the pulse sequence: repetition/echo time TR/TE = 550/13 ms, excitation angle α = 90°, refocusing angle β = 180°, inter-echo time IET = 13 ms, echo train length ETL = 4.
-
Combined Echo Two-Point Dixon method for high efficiency water/fat separation
Shi Cheng1, Kun Zhou1, Wei Liu1, and Dehe Weng1
1Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China
An imaging method utilizing two pairs of fast-switching bipolar
readout gradients and partially-opposed-phase and in-phase Dixon (CETD) is
proposed to further reduce dead time. The novel steps in
joint reconstruction of the two pairs ensure the consistency of water-fat
separation.
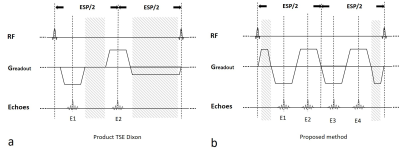
Figure 1. The diagram in one echo spacing of the TSE Dixon
method (a) and proposed combined echo two-points Dixon sequence (b). The dead
time is marked with diagonal stripes, where no data sampling happens. In the
proposed method, the dead time is reduced. Note that E1 and E4 are partially
opp-phase echoes, while E2 and E3 are partially in-phase echoes.
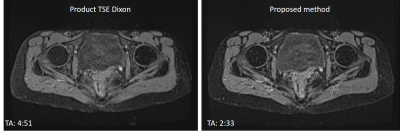
Figure 4. Water images of a volunteer acquired with product TSE
Dixon (left) and combined echo two-points Dixon (right). SNR and image quality
are comparable.
-
Neuronal current imaging on clinical whole body scanners: capabilities and limitations
Milena Capiglioni1, Claus Kiefer2, and Roland Wiest1
1Institute for Diagnostic and Interventional Neuroradiology, Support Center for Advanced Neuroimaging (SCAN), University of Bern, Bern, Switzerland, 2Institute for Diagnostic and Interventional Neuroradiology, Support Center for Advanced Neuroimaging (SCAN), Inselspital, Bern, Bern, Switzerland
We detected oscillating fields in the nT range using a new Spin lock based contrast. This study takes a further step towards the use of neuronal current imaging in clinical applications.
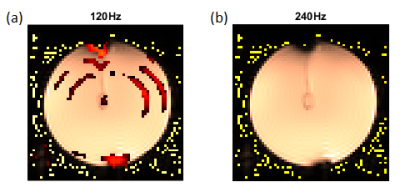
Figure 5: Double resonance effect at two different frequencies of the Spin locking (a) 120 Hz and (b) 240 Hz. For both images, the frequency of the applied current was set at 120 Hz and the amplitude was calculated by the Biot-Savart law to be approximately 10 nT. To facilitate the visualization of the results, the rest of the deconvolution between SL on and SL off is displayed.
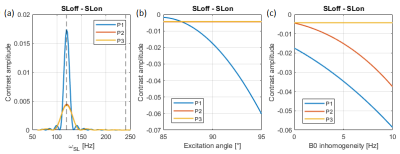
Figure 3: Bloch simulation of the behaviour of the three SL approaches showed in Figure 1. a) Contrast as a function of the SL frequency for homogeneous fields. b) Contrast in resonance (120 Hz) for the three approaches as a function of the excitation angle, where the variation is induced by B1 inhomogeneity. c) Contrast in resonance (120 Hz) for the three approaches as a function of the off resonant frequency induced by B0 inhomogeneity.
-
Quantifying lactate using double quantum filtered 1H MRS with adiabatic refocusing pulses at 7 T
Fabian Niess1, Albrecht Ingo Schmid1, Graham Kemp2, Ewald Moser1, Maxim Zaitsev1, and Martin Meyerspeer1
1High Field MR Center, Center for Medical Physics and Biomedical Engineering, Medical University Vienna, Vienna, Austria, 2Department of Musculoskeletal & Ageing Science, Institute of Life Course and Medical Sciences, University of Liverpool, Liverpool, United Kingdom
Double quantum filtered 1H MRS using adiabatic refocusing pulses at 7 T was successfully implemented. Fibre orientation-dependent quantification of lactate in meat for four different, clinically relevant angles relative to B0 was demonstrated.
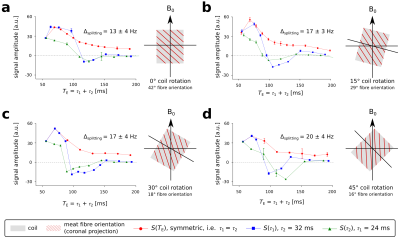
Integrated signal area of the lactate doublet at 1.3 ppm as a function of the echo time (TE = τ1 + τ2). The coloured lines represent different combinations of τ1 and τ2 to achieve the effective TE with either τ1 = τ2 (red), holding τ2 constant (blue) or τ1 constant (green). The measurements were repeated with four different orientations of the RF coil (grey box) relative to the main magnetic field (0° to 45°) with estimated fibre orientations ranging from 42° to 16° (projections to the coronal plane hatched in red).
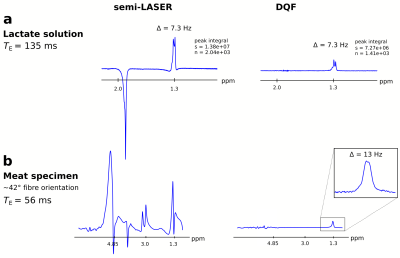
Averaged 1H MR spectra (n = 30, TR = 3 s) acquired using semi-LASER (left) and with the adiabatically refocused DQF acquisition scheme (right). Spectra were acquired from
(a) lithium lactate solution in a spherical phantom (d = 160 mm, 96mM LiLac in H2O) and (b) sodium lactate (60 %) injected into a pork meat specimen. Only lactate is visible (at 1.3 ppm) in spectra from both experiments with a peak splitting of 7.3 Hz for lactate in solution and 13 Hz for lactate injected in meat.
-
Increasing three-dimensional coverage of dynamic speech magnetic resonance imaging
Riwei Jin1, Zhi-pei Liang1, and Bradley P. Sutton1
1University of Illinois Urbana-Champaign, Champaign, IL, United States
We increase the 3D coverage of dynamic speech MRI to 32 slices with 96 mm full thickness with 1.875×1.875× 3mm and temporal resolution of 35 fps by applying sparsly sampled navigators and Partial-Separability model which makes us approahing isotropic resolution with full vocal tract.
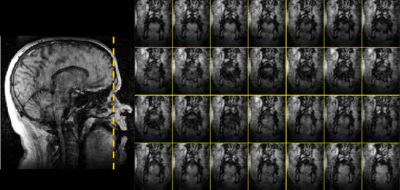
Figure 3 Coronal images
of mid-tongue region in 28 frames (about 0.8s) of subject counting ‘Four’
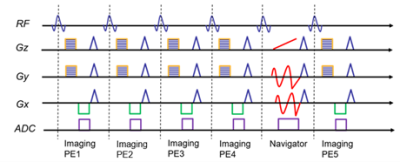
Figure 1. A simplified pulse sequence diagram illustrating (k,
t)-space sampling patterns. The navigator dataset is acquired using a spiral
trajectory and is only acquired once every 4 imaging data acquisitions. The
imaging dataset is acquired using a Cartesian trajectory with random phase and
slice encoding to provide structural quality images.
-
Multi-Slice 2D pTx Readout-Segmented Diffusion-Weighted Imaging Using Slice-by-Slice B1+ Shimming
Sydney Nicole Williams1, Iulius Dragonu2, Patrick Liebig3, and David A. Porter1
1Imaging Centre of Excellence, University of Glasgow, Glasgow, United Kingdom, 2Siemens Healthcare Ltd., Frimley, United Kingdom, 3Siemens Healthineers, Erlangen, Germany
We demonstrate slice-by-slice pTx shimming in a multi-slice readout-segmented diffusion-weighted sequence at 7 tesla. Slice-by-slice shimming is simple and efficient while greatly improving flip-angle homogeneity compared to volumetric-shimming and single-transmit.
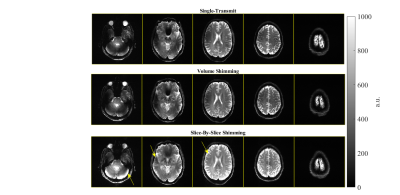
Figure 4. Experimental readout-segmented 2D images in a healthy subject: Top row) single-transmit, Middle row) volumetric-B1+ shimming, and Bottom row) slice-by-slice B1+ shimming. The arrows highlight a few areas where slice-by-slice shimming mitigates the B1+ inhomogeneity particularly well compared to single-transmit and volumetric-shimming. Meanwhile, there is a global improvement in the column 4 slice. Each column of slice images are windowed the same.
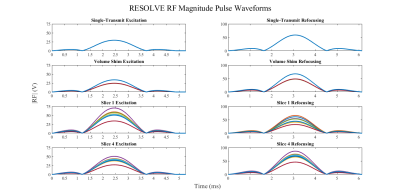
Figure 2. Magnitude RF waveforms in volts for 8 transmit channels, excitation pulse on the left and refocusing pulse on the right. Top row) single-transmit waveforms (all channels same magnitude with incremental 45° phase offset); Second row) volumetric-shim waveforms (in this case, the shimmed solution alternated between two magnitude values on all channels); Third row) slice 1 shim waveforms; Fourth row) slice 4 shim waveforms.
-
Simultaneous Measurements of Low-b Spin-Echo and High-b Stimulated-Echo DWI for Ultrahigh-b DWI
Kyle Jeong1, Noel Carlson2, John Rose3, and Eun-Kee Jeong4
1Utah Center for Advanced Imaging Research, University of Utah, Salt Lake City, UT, United States, 2NeuroImunology and NeuroVirology, University of Utah, Salt Lake City, UT, United States, 3Neurology, University of Utah, Salt Lake City, UT, United States, 4Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States
DWSE and DWSTE were successfully combined for ultrahigh-b DWI of CSC imaging. The combined data set provides reliable signal-b curves for further quantitative analysis. The imaging time was only 6 min for 21 slices, bmax of 9781 and 3240 s/mm2 with 13 and 7 b-values for rDWI and aDWI, respectively.
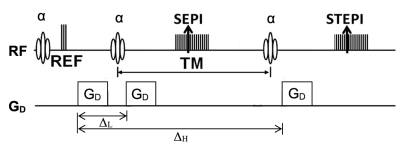
Fig. 1. Pulse sequence diagram of DW-SESTE with the flipangle α and amplitude GD and duration δ of the diffusion gradients.
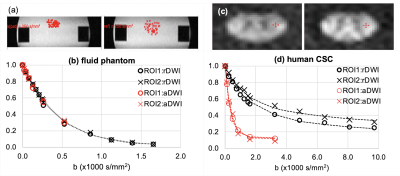
Fig. 2. (a, c) ROI (red dots and crosses) and (b, d) signal-b curves of UHb-DWI. Signal-b curves were fit to a single-exponential function for the phantom data in (b) and human aDWI data (red O, X)) in (d), and a double-exponential function for human rDWI data (black O, X) in (d).
-
Simultaneous T1, T2 and T2* Mapping of the Carotid Plaque Using Combined Single- and Multi-echo 3D Golden Angle Radial Acquisition
Yajie Wang1, Yishi Wang2, Haikun Qi3, Rui Guo4, Huiyu Qiao1, Dongyue Si1, and Huijun Chen1
1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 2Philips Healthcare, Beijing, China, 3School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 4Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, United States
A
new quantitative technique using combined single- and multi-echo 3D golden angle
radial acquisition for simultaneous T1, T2 and T2* mapping of the carotid
plaque has been demonstrated to have good quantitative accuracy and in-vivo
feasibility.
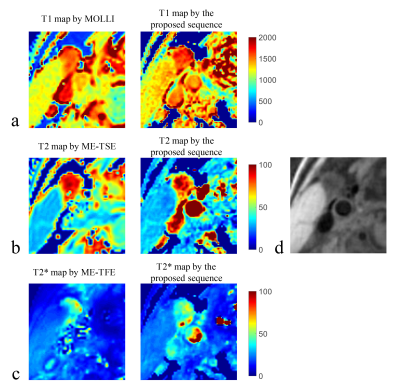
Figure
3. Quantitative mapping results of one healthy volunteer (male, 30 years). (a)
T1 maps estimated by modified Look-Locker inversion recovery
(MOLLI) and
the proposed sequence; (b) T2
maps
estimated by multi-echo
tubor spin
echo (ME-TSE)
and the proposed sequence;
(c) T2* maps
estimated by multi-echo
turbo
field
echo (ME-TFE)
and the proposed sequence;
(d) vessel wall image reconstructed from the proposed sequence.
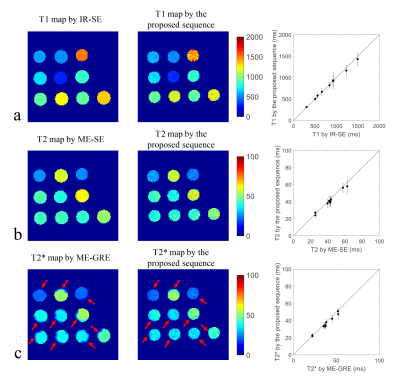
Figure
2. Quantitative mapping results of the
phantom. (a) T1 maps estimated
by
inversion
recovery spin echo (IR-SE)
and the proposed sequence with quantitative comparison (mean and SD); (b)
T2
maps
estimated
by
multi-echo
spin echo (ME-SE) and the proposed sequence with quantitative comparison (mean
and SD); (c)
T2*
maps
estimated
by
multi-echo
gradient echo (ME-GRE) and the proposed sequence with quantitative comparison
(mean and SD).
-
Optimization of Magnetization Transfer Contrast for EPI FLAIR Brain Imaging
Serdest Demir1, Bryan Clifford2, Thorsten Feiweier3, Tom Hilbert4, Zahra Hosseini5, Augusto Lio Goncalves Filho1, Azadeh Tabari1, Wei-Ching Lo2, Maria Gabriela Figueiro Longo1, Michael Lev1, Pamela Schaefer1, Otto Rapalino1, Kawin Setsompop6, Berkin Bilgic6, Stephen Cauley6, Susie Huang1, and John Conklin1
1Radiology, Massachusetts General Hospital, Boston, MA, United States, 2Siemens Medical Solutions, Boston, MA, United States, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Siemens Healthcare AG, Lausanne, Switzerland, 5Siemens Medical Solutions, Atlanta, GA, United States, 6Radiology, A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, MA, United States
EPI FLAIR images have lower
tissue contrast than TSE FLAIR images due to the absence of significant MT
effects. We demonstrate that an optimized MT-prepared EPI FLAIR acquisition can
achieve equivalent tissue contrast to conventional TSE FLAIR images.
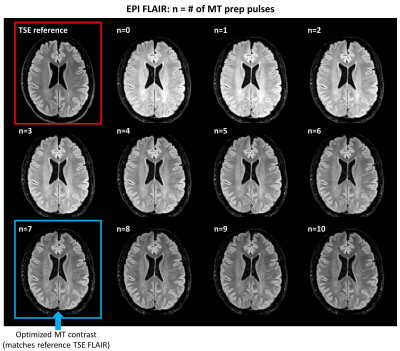
Figure 2. Representative images showing the effect of additional
magnetization preparation pulses (f=1200 Hz, a=1.0) on EPI FLAIR contrast
compared to the clinical reference TSE FLAIR scan (top left). Optimal
EPI FLAIR contrast (i.e., most closely matching the TSE reference scan) was
achieved with 7 preparation pulses (see Figure 4 for quantitative evaluation).
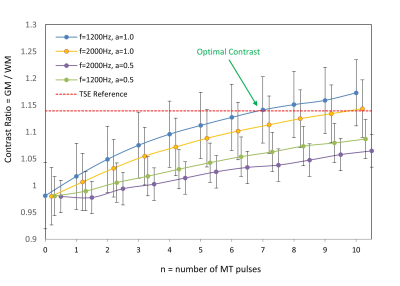
Figure 4. Contrast ratio for EPI
FLAIR images (defined as average GM / WM signal) as a function of the number of
MT preparation pulses (n), resonance offset (f), and pulse amplitude (a).
Contrast ratio for the TSE reference scan is shown by the dashed red line.
Optimal contrast (i.e., most clostly matching the TSE reference contrast) was
achieved with n=7, f=1200Hz, and a=1.0.
-
A Short TR Adiabatic Inversion Recovery Zero Echo Time (STAIR-ZTE) Sequence with Interleaved Encoding and a Modulated RF Pulse for Myelin Imaging
Hyungseok Jang1, Yajun Ma1, Michael Carl2, Saeed Jerban1, Roland Lee1, Eric Y Chang1,3, Jody Corey-Bloom1, and Jiang Du1
1University of California, San Diego, San Diego, CA, United States, 2GE Healthcare, San Diego, CA, United States, 3Veterans Affairs San Diego Healthcare System, San Diego, CA, United States
In this study,
we investigated feasibility and efficacy of the proposed STAIR-ZTE sequence for
direct volumetric myelin imaging in human brain, which may provide an MR
biomarker for diagnosis and monitoring of demyelinating disorders.
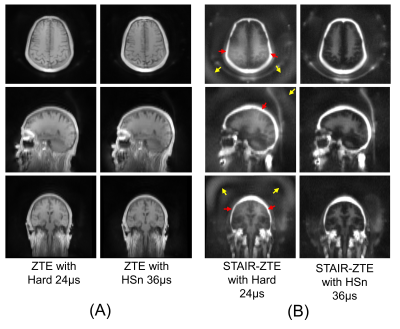
Figure 3. Efficacy of HSn pulse (47-year-old male). (A) ZTE
imaging without IR and (B) STAIR-ZTE. A regular ZTE does not show a dramatic
difference between images with a hard pulse and an HSn pulse because the excitation bandwidths of both hard and HSn pulses are broad enough
to include the targeted region. STAIR-ZTE shows dramatic improvement with an
HSn pulse, where the low frequency signal bias (yellow arrows) affecting
myelin contrast (red arrows) is well suppressed. With suppression of long T2
signals, the remaining myelin signal becomes much lower and prone to imaging
artifacts.
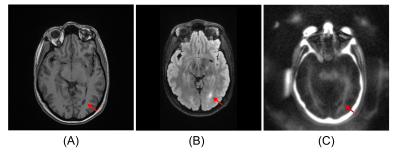
Figure 5. A representative MS patient (22-year-old
female). (A) MP-RAGE, (B) FLAIR, and (C) STAIR-ZTE. STAIR-ZTE delineates a
demyelinated lesion which corresponds well with the clinical MP-RAGE and FLAIR
images (red arrows).
-
Knee osteochondral junction imaging using a 3D dual adiabatic inversion recovery ultrashort echo time cones (3D DIR-UTE-cones) sequence at 3T
Alecio F. Lombardi1,2, Zhao Wei1, Hyungseok Jang1, Saeed Jerban1, Lillian Gong1, Jiang Du1, Eric Y. Chang1,2, and Ya-Jun Ma1
1Radiology, University of California, San Diego, CA, United States, 2Radiology Service, Veterans Affairs, San Diego, CA, United States
The 3D-DIR-UTE-Cones sequence can produce high-resolution and high-contrast imaging of the OCJ region
of the knee in vivo and shows more efficient subchondral bone marrow fat suppression compared to the IR-FS-UTE Cones sequence.
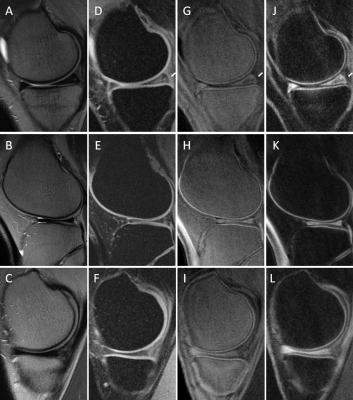
Figure 3. T2w-FSE (A, B, C), FSPGR (D, E, F), 3D IR-FS-UTE
Cones (G, H, I), and 3D DIR-UTE-Cones (J, L, M) performed in knees of two healthy
volunteers (the first row represent the first volunteer). On T2w-FSE and FSPGR,
the signal in the OCJ region cannot be detected due to its fast signal decay. (G-I)
3D IR-FS-UTE Cones sequences highlighting the OCJ and suppressing SC and
subchondral bone. (J-L) 3D DIR-UTE-Cones sequences also highlight the OCJ but
with better subchondral bone fat suppression.
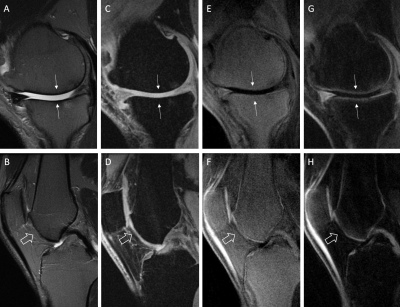
Figure 4. T2w-FSE (A, B), FSPGR (C, D), 3D IR-FS-UTE Cones
(E, F), and 3D DIR-UTE-Cones (G, H) performed in the knees of two patients with
osteoarthritis. (Arrows in A, C, E, G) show interruption of the bright-line
representing OCJ on the weight-bearing surface of the medial femoral condyle
and medial tibial plateau in the first patient and on the femoral trochlea in
the second patient (open arrows in B, D, F, H). The 3D IR-UTE-FS Cones
sequences (G and H) are more efficient in suppressing the subchondral bone fat
than the 3D IR-FS-UTE Cones (E, F).
-
Phantom validation of a novel user-configurable ultrashort echo time (UTE) sequence
Lumeng Cui1, Emily J. McWalter1,2, Gerald R. Moran3, and Niranjan Venugopal4
1Division of Biomedical Engineering, University of Saskatchewan, Saskatoon, SK, Canada, 2Department of Mechanical Engineering, University of Saskatchewan, Saskatoon, SK, Canada, 3Research Collaboration Manager, Siemens Healthcare Limited, Oakville, ON, Canada, 4Department of Radiology, University of Manitoba, Winnipeg, MB, Canada
The UTE sequence developed allows for 2D and 3D acquisitions, choice of trajectory, off-resonance artifact reduction, RF pulse choice and long T2 species suppression, as such it is a flexible tool that can be used and optimized for many UTE applications.
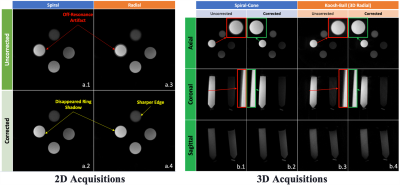
Figure 2. The off-resonance artifact improved with the deblurring algorithm in both the 2D Spiral (a.1-2) and the 2D Radial (a.3-4) acquisitions. The off-resonance artifact improved with the deblurring algorithm in both the 3D Spiral-cone (b.1-2) and 3D Radial (“Koosh-Ball”) (b.3-4) acquisitions. The red and green enlargements in figure b highlight the comparison before and after the correction in 3D. Beginning from the top and going clockwise, the vials contain rubber, water, agar and oil.
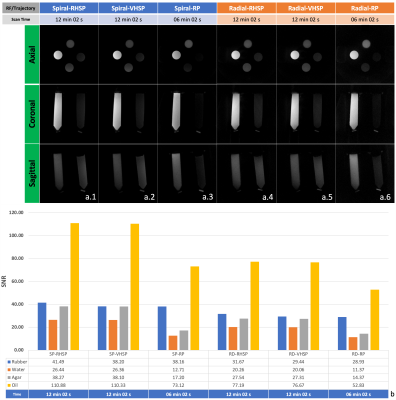
Figure 4. Comparison of 3D radial and spiral trajectories among the regular half-Sinc pulse (RHSP) excitation, VERSE-modified half-Sinc pulse (VHSP) excitation, and the rectangular pulse (RP) excitation with matching scan parameters (a.1-6). SNR of the rubber (short T2 material) was generally higher for the spiral trajectories as compared to the radial trajectories. SP-spiral; and RD-radial. Beginning from the top and going clockwise, the vials contain rubber, water, agar and oil.
-
Volume-Selective 3D Ultrashort Echo-time Imaging
Jinil Park1 and Jang-Yeon Park2,3
1Biomedical Institute for Convergence at SKKU, Sungkyunkwan University, Suwon, Korea, Republic of, 2Department of Biomedical engineering, Sungkyunkwan University, Suwon, Korea, Republic of, 3Department of Intelligent Precision Healthcare Convergence, Sungkyunkwan University, Suwon, Korea, Republic of
this study demonstrates VS-UTE ability to maintain image quality when the number of projection views is undersampled or when the imaging volume is selected smaller than the imaging target.
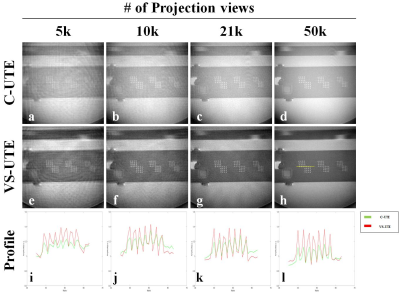
Figure 3. the images including the resolution grids and 1D profiles of the ACR phantom acquired
using conventional UTE (C-UTE) (a-d) and VS-UTE (e-h). The C-UTE image is
difficult to show the internal structure due to the phantom signal outside the
ROI when the number of projections was 5k, but VS-UTE provided a better internal structure image. Also, the
overall CNR (contrast to noise ratio) of C-UTE was lower than that of
VS-UTE(i-l).
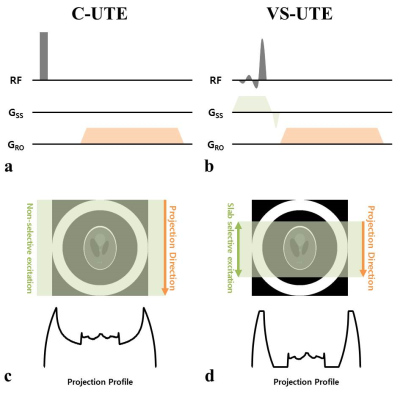
Figure 1. Pulse sequence diagrams(a, b)
and data acquisition method(c, d) of conventional UTE (C-UTE, a) and
volume-selective UTE (VS-UTE, b). C-UTE, which uses a non-selective pulse for
spin excitation(a), acquires projection data including spin information of the
entire object(c). In contrast, The spin excitation of the unexcited object is
not included in the signal(d) by using the slab selective pulse and the slab
selective gradient simultaneously and setting the projection direction in the
same direction as the slab selection direction(b).
