-
Comparison of pharmacokinetic models for assessing murine renal function by DCE-MRI
Soham Mukherjee1, Mahon L Maguire1, Jack Sharkey1, Sourav Bhaduri1, Patricia Murray2, Rachel Bearon3, Bettina Wilm2, and Harish Poptani1
1Centre for Preclinical Imaging, University of Liverpool, Liverpool, United Kingdom, 2Department of Cellular and Molecular Physiology, University of Liverpool, Liverpool, United Kingdom, 3Department of Mathematical Sciences, University of Liverpool, Liverpool, United Kingdom
Dynamic contrast enhanced magnetic resonance
imaging was used to determine the permeability parameter Ktrans, to
assess renal function.
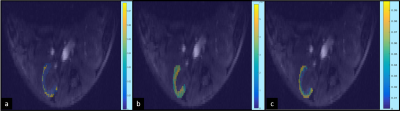
Fig. 1: Ktrans (s-1) maps of the cortical region overlaid on the left kidney of a mouse. The raw AIF
was used to compute the Ktrans maps using the non-linear Tofts (a), extended
Tofts (b), and the SSM (c).

Fig. 2: Boxplot of mean Ktrans (s-1)values
from fitting of nonlinear Tofts, extended Tofts and SSM using AIF derived from
(a) raw data, (b) SSA denoising, and (c) bi-exponential fitting.
-
Diffusion Time Dependence of Apparent Diffusion Coefficient and Intravoxel Incoherent Motion Diffusion Parameters in the Human Kidney
Julia Stabinska1, Hans-Joerg Wittsack1, and Alexandra Ljimani1
1Department of Diagnostic and Interventional Radiology, Heinrich Heine University Dusseldorf, Dusseldorf, Germany
Mono-exponential ADC and IVIM-related diffusion coefficient D of the kidney slightly increase with diffusion time as opposed to ADC of skeletal muscle. Pseudodiffusion coefficient D* shows the strongest dependence on diffusion time among the investigated parameters.
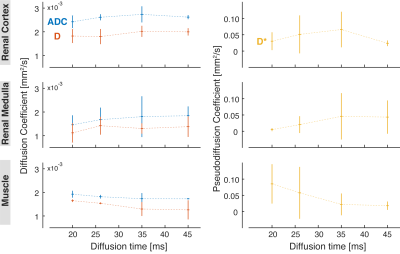
Mono-exponential ADC and IVIM-related parameters (D, D*) measured in the renal cortex (top row), renal medulla (middle row) and skeletal muscle (bottom row).
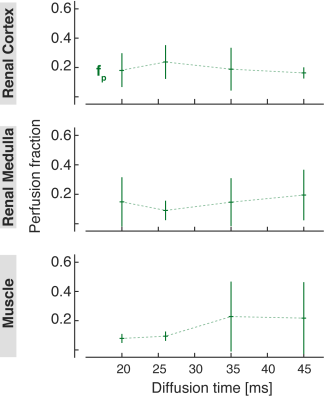
Perfusion fraction fp obtained from the IVIM analysis in the renal cortex (top row), medulla (middle row), and skeletal muscle (bottom row).
-
Phosphorus Magnetic Resonance Spectroscopy of Healthy Human Kidney in-situ at 3T
Maysam Jafar1 and Jan Weis2
1Clinical Science, Philips Healthcare, Stockholm, Sweden, 2Department of Medical Physics, Uppsala University Hospital, Uppsala, Sweden
·
31P-MRS of normal human kidney in-situ is feasible on 3T clinical MR systems in an acceptable measurement time.
·
The amplitudes of b-ATP resonances are decreased due to the narrowing of the
effective excitation bandwidth with respect to distance from the surface coil.
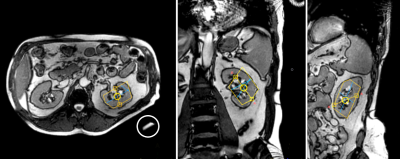
Figure 1: Typical voxel position in axial,
coronal and sagittal planes. A small water-containing bottle (circled) was
attached to the centre of the coil as a marker of coil position.
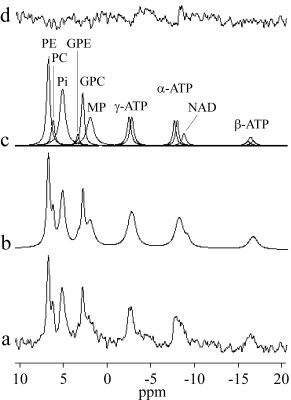
Figure 2: 31P spectrum of the normal human
kidney in situ. (a) The added spectrum of the five healthy volunteers, (b)
fitted spectrum, (c) individual components, and (d) residue.
-
Repeatability of multi-parametric renal MRI biomarkers in healthy subjects: An iBEAt pilot study
Kanishka Sharma1, Bashair Alhummiany2, David Shelley2,3, Margaret Saysell2,3, Maria-Alexandra Olaru4, Bernd Kühn4, Julie Bailey3, Kelly Wroe3, Cherry Coupland3, Michael Mansfield3, and Steven Sourbron1
1Department of Imaging, Infection, Immunity and Cardiovascular Disease, University of Sheffield, Sheffield, United Kingdom, 2Department of Biomedical Imaging Sciences, University of Leeds, Leeds, United Kingdom, 3Leeds Teaching Hospitals, Leeds, United Kingdom, 4Siemens Healthcare GmbH, Erlangen, Germany
The results indicate overall
comparable repeatability for MRI biomarkers of renal tissue structure and perfusion
using phase contrast, while also highlighting the need for formal MRI quality
assurance prior to image processing.
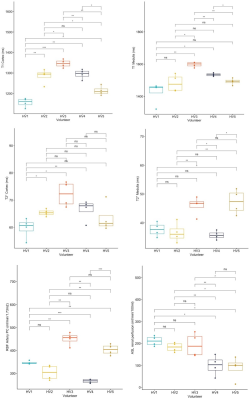
Figure 3. Box plots for T1, T2*
mapping in the renal cortex and medulla (ROIs), arterial RBF (BSA normalised)
using PC-MRI, and renal perfusion (ml/min/100ml) with ASL, from 4 repeatability
measurements in 5 healthy volunteers (HV) on the reference MRI scanner (MAGNETOM Prisma 3T, Siemens
Healthcare GmbH, Erlangen, Germany) using the iBEAt MRI protocol. Pairwise comparison using
t-test shows the statistical significance of differences (ns: not significant =
p > 0.05; *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001) between HVs.
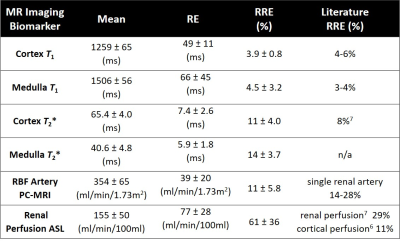
Table 1. 95% confidence interval for the mean value of each
parameter (1st column), and for the mean value of their RE (2nd
column) and RRE (3rd column). Literature values of RRE (4th column).
-
Measurement of renal medullary perfusion using a 7-compartment model for MR Renography
Anneloes de Boer1, Bashair Al Hummiany2, Kanishka Sharma3, and Steven Sourbron3
1University Medical Center Utrecht, Utrecht, Netherlands, 2University of Leeds, Leeds, United Kingdom, 3University of Sheffield, Sheffield, United Kingdom
A 7-compartment model was developed to measure medullary
perfusion using MR renography and validated using simulations. In diabetic
patients, the model produced relatively high medullary perfusion values of 81 mL/100mL/min.
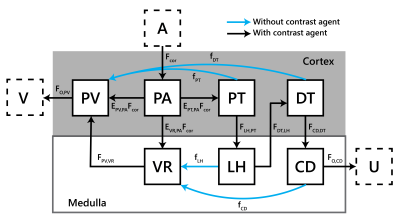
Figure 1: The
7 compartment model;
Fmed=EVR,PAFcor
. A aorta;
V renal veins; PV venous plasma compartment (low pressure vascular spaces
including veins and peritubular capillaries); PA arterial plasma compartment
(high pressure vascular spaces including arteries and glomeruli); PT proximal
tubules; DT distal tubules; VR vasa recta; LH loop of Henle; CD collecting ducts; U urine. The blue arrows
represent reabsorption flows carrying mainly water but no contrast agent.
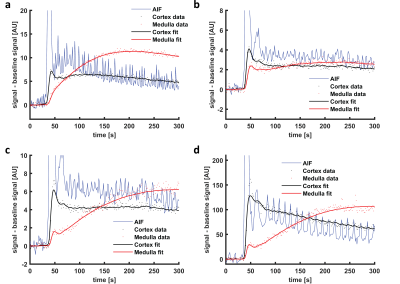
Figure 3: Example
of a and b) a 7CM fit to simulated
data and c and d) a 7CM fit to
patient data (only the first 300 s are shown). Note that the model fits the
first pass peak well in simulated data, while it is not capable to capture the
full height of the first pass peak in patient data. The oscillations on the AIF
are due to inflow effects.
-
Improved Accuracy of Ratiometric CEST pH Mapping using Two Iodinated Agents with Nonequivalent Amide Protons and a Single Low Saturation
Quan Tao1, Peiwei Yi1, Zimeng Cai1, Yingjie Mei2, Ruiyuan Liu1, and Yanqiu Feng1
1School of Biomedical Engineering, Southern Medical University, Guangzhou, China, 2Philips healthcare, Guangzhou, China
The combination of
iobitridol and iodixanol with the mixed ratio of 1:1 was found to be the best
for pH mapping. Improved accuracy and extended pH detection range have been
achieved under a single reduced B1 of 1.5 μΤ, which enable the
reliable pH mapping of kidney in vivo.
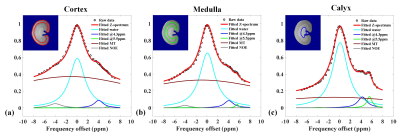
Figure
4. In vivo Z-spectra of cortex (a), medulla (b) and calyx (c) in one rat kidney
after intravenous injection of iodixanol and iobitridol mixture, and the
fitting results using the multi-pools Lorentzian model .
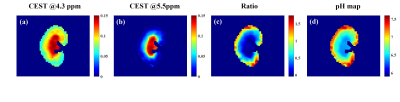
Figure 5. Resolved
maps of two CEST effects at 4.3 ppm (a) and 5.5 ppm (b) obtained from acquired
Z-spectra of one rat kidney under a saturation power of 1.5 μT; Ratiometric
image (c) and the pH map (d) obtained from the two CEST effects.
-
Effect of gravity on kidney function: evaluation using multiposture MRI
Yuki Oda1, Tosiaki Miyati1, Naoki Ohno1, Seiya Nakagawa1, and Satoshi Kobayashi1
1Division of Health Sciences, Kanazawa University, Kanazawa, Japan
Gravity reduces the
blood flow and T2 of the kidney. Multiposture MRI makes it possible
to evaluate the effect of gravity on regional kidney function.
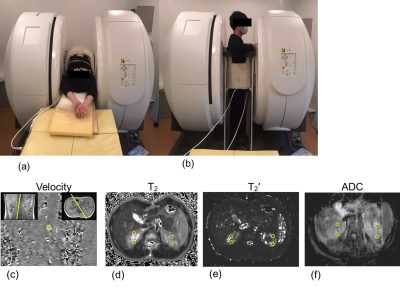
Figure 1. (a) Supine and (b) upright positions in the multiposture
MRI. Regions of interest (yellow open circles) on the (c) velocity, (d) T2,
(e) T2’, and (f) apparent diffusion coefficient (ADC) images.

Figure 2. Mean
renal blood flow of the (a) right and (b) left kidneys, and (c) heart rate in
the supine and upright positions.
-
BOLD MRI for evaluating intra-renal oxygenation level during acute saline loading
El-Sayed H Ibrahim1, Abdul Parchur1, Srividya Kidambi1, Allen Cowley1, and Mingyu Liang1
1Medical College of Wisconsin, Milwaukee, WI, United States
BOLD MRI is capable of monitoring acute changes in the regional
hemodynamics within the kidney. The variable responses seen in the imaged
subjects may reflect differences in salt-sensitive versus salt-insensitive
individuals
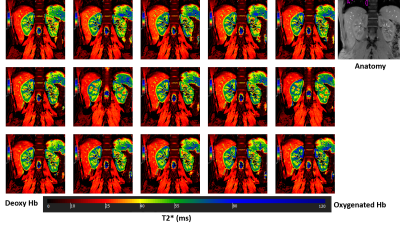
Fig 1. Sequential T2* BOLD images acquired in a volunteer during one hour of
saline infusion. An anatomical image is shown for reference. The images show
clear gradient in tissue oxygenation level between cortex and medulla,
represented by higher and lower T2* values, respectively, based on the T2*
color map (measured in ms).
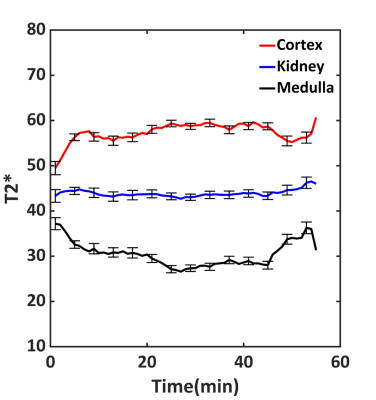
Fig 3. Average T2* values (ms) from all volunteers during one hour of
saline infusion. Average T2* in the kidney was constant, while T2* values in
the cortex were significantly higher than those in the medulla. Note changes in
T2* values at the beginning and end of the experiment when infusion rate was
lower than the rest of the experiment.
-
Severity of Tubular Atrophy and Fibrosis in Acute Kidney Injury Revealed by Multi-parametric MRI
Feng Wang1,2, Tadashi Otsuka3, Zhongliang Zu1,2, Mark P de Caestecker3, Raymond C Harris3, Takamune Takahashi3, and John C Gore1,2
1Vanderbilt University Institute of Imaging Science, Vanderbilt University Medical Center, Nashville, TN, United States, 2Department of Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States, 3Division of Nephrology and Hypertension, Vanderbilt University Medical Center, Nashville, TN, United States
MRI measures such as pool size ratio, relaxation rates, and parameters derived from R1ρ dispersion are sensitive to tubular atrophy and fibrosis in kidneys. Spin-lock MRI provides parameters with higher sensitivity than R1 and R2.
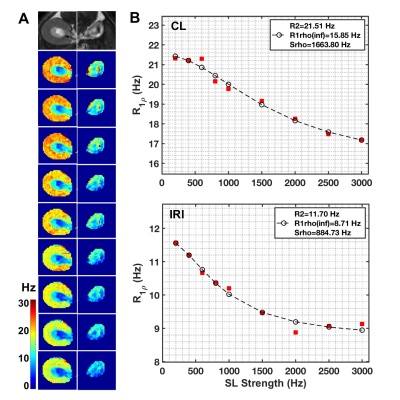
Figure 4. Comparison of representative in vivo R1ρ dispersion between CL and IRI kidneys. (A) T2-weighted images (T2W) and R1ρ maps at different spin-locking strength with locking frequency from 200 to 3000 Hz (top to bottom). (B) Comparison of R1ρ dispersions between CL and IRI kidneys. The parameters were derived from Chopra model, with fitting results from SL strength ranges 200-3000 Hz shown. Cortex and OSOM were included to calculate the averaged R1ρ at each spin-lock strength for further fitting.
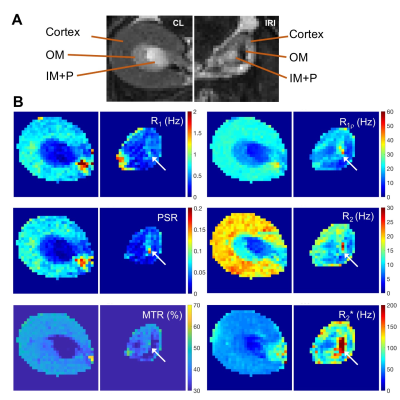
Figure 5. Representative comparison of MRI images and maps of CL and IRI kidneys. (A) T2-weighted (T2W) images zoomed on CL and IRI kidneys. (B) R1, R1ρ (spin-lock frequency 1000 Hz), R2, R2*, pool size ratio (PSR) from quantitative magnetization transfer (qMT) modeling, and magnetization transfer ratio (MTR) based on images without and with magnetization transfer saturation (flip angle 820 degree and RF offset 5000 Hz). The arrows indicate outer medulla.
-
Detection of fibrosis in patients with moderate renal impairment with multiparametric MRI
Pete Thelwall1,2, Jehill Parikh1, Benjamin Pippard1, Caroline Wroe3, Rob Janiczek4, Steven Sourbron5, and Neil Sheerin1
1Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne, United Kingdom, 2Centre for In Vivo Imaging, Newcastle University, Newcastle upon Tyne, United Kingdom, 3South Tees Hospitals NHS Foundation Trust, Middlesborough, United Kingdom, 4GlaxoSmithKline, Philadelphia, PA, United States, 5University of Sheffield, Sheffield, United Kingdom
A multiparametric kidney scan protocol was implemented. An increase in cortical native T1 and T2 was observed in patients with moderate renal impairment and biopsy-proven fibrosis compared to healthy controls.
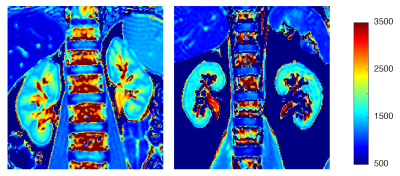
T1 maps from a healthy volunteer (left) and patient with moderate renal impairment and biopsy-proven fibrosis (right). Lower contrast (ie. smaller T1 different) between cortical and medullary regions is observed in the patient with renal disease compared to the healthy volunteer.

Cortical and medullary T1 (pre- and post-administration of gadolinium-based contrast agent), T2 and T2* measurements from healthy volunteers and patients with moderate renal impairment. ‡ denotes a statistically significant different from healthy controls (p < 0.05).
-
Renal lipid content based on PDFF Imaging is a new potential biomarker for assessing early renal injury in patients with metabolic syndrome
Shisi Li1, Yanjun Chen1, Yingjie Mei2, Xianfu Mo1, Jialing Chen1, Yongqiang Li3, and Xiaodong Zhang1
1Department of Medical Imaging, The Third Affiliated Hospital of Southern Medical University, Guangzhou, China, 2Philips Healthcare, Guangzhou, China, 3Department of Nephrology, The Third Affiliated Hospital of Southern Medical University (Academy of Orthopedics· Guangdong Province), Guangzhou, China
Renal fat content has been known as correlated with
renal injury in type 2 diabetes. However, it is not clear the change of renal
fat content in patients
with metabolic syndrome (MS), which is a more popular disease threatened human
health.
In
the present study, we assess the feasibility and reproducibility of renal fat
fraction (FF) using PDFF imaging with MR mDixon-Quant sequence. And we aim to investigate the changes of renal FF in patients with
MS, whose estimated glomerular filtration rate (eGFR) grade were G1(normal or
elevated) and G2(mild decline) described in KDIGO (Kidney Disease: Improving
Global Outcomes). In addition, we evaluate the correlation of renal FF and eGFR
and the major factors of eGFR. The results show that with eGFR decreasing, renal
FF in patients with MS-G2 group increased significantly compared with control
and MS-G1 group. And the renal FF is an important affection factor of eGFR with
a significant negative correlation.
The noninvasive quantitative Dixon-based MRI may be a new biomarker for
the evaluation of early renal impairment.
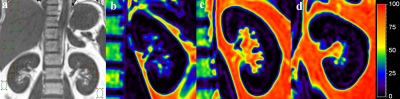
Fig.1 Regions
of interest were drawn manually on the kidney,
liver and perirenal fat
to obtain the quantitative FF(a). Colour-coded
FF maps from a 25-year-old non-
Metabolic syndrome male (b) , a 28-year-old male in MS-G1 group
(c),
and a 40-year-old male
in
MS-G2 group (d),
whose
average renal FF are 3.34%,
4.41%
and 5.71% respectively .
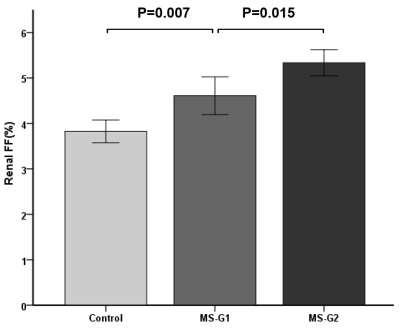
Fig.2 In groups of control, MS-G1 and MS-G2, renal FF increased gradually and with significant differences among the groups(P<0.01).
-
Explore the performance of FF and R* value measured by mDIXON-quant for heathy controls, mild and acute CKD patients.
Haoyang Jiang1, Ailian Liu2, Ye Ju2, Jiazheng Wang3, Changyu Du1, Lingli Qi1, Xinmiao Bu2, Wenjun Hu2, Nan Wang2, and Liangjie Lin3
1Dalian Medical University, Dalian, China, 2The First Affiliated Hospital Of Dalian Medical University, Dalian, China, 3Philips Healthcare, Beijing, China
In summary, the R2* value
and fat fraction by mDIXON-Quant may help clinical diagnosis of CKD with
quantitative evaluation of hypoxia and lipid deposition in renal tissues.
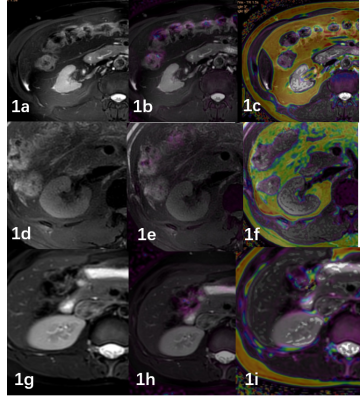
Fig1. A 50-year-old
male with
CKD grade 5,eGFR
was 7.87ml/min (1a).
T2WI image. (1b)R2* image (1c).
FF
image.
A 40-year-old
male
with CKD grade 1,eGFR
was 124.09ml/min (1d).
T2WI image.
(1e)R2*
image (1f).
FF
image
A 31-year-old female volunteer (1g).
T2WI image.
(1h)R2*
image (1i).
FF
image
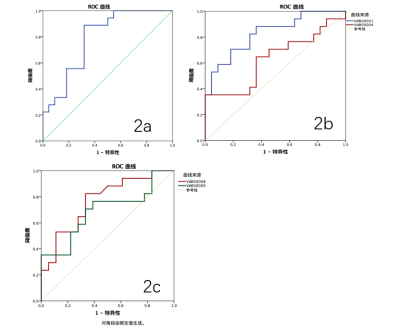
Fig2. Diagnostic efficiency curve of the R2* values in heathy controls and mild CKD
patients (2a).
Diagnostic efficiency curve of the R2* values in heathy controls and acute CKD
patients (2b).
Diagnostic efficiency curve of the FF values in mild CKD patients and acute CKD
patients (2c).
-
Value of quantitative susceptibility mapping for detecting renal fibrosis of early diabetic nephropathy in type 2 diabetes
Jiayuan Shan1, Jinggang Zhang1, Jie Chen1, Wei Xing1, and Jilei Zhang2
1Radiology, Third Affiliated Hospital of Soochow University, Changzhou, China, 2Philips Healthcare, Shanghai, China
The purpose was to explore if quantitative susceptibility mapping (QSM) can assess renal fibrosis about early diabetic nephropathy (DN) in type 2 diabetes (T2D).
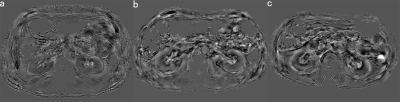
Susceptibility
maps of both kidneys for DN stage Ⅰ(a), Ⅱ(b) and Ⅲ(c). As the stage of DN
increased, the medulla showed stronger diamagnetic value. However, there was no
significant changes in the cortex.
-
Multiparametric MR imaging in diabetic nephropathy: New insights to evaluate early diabetic nephropathy noninvasively
Akira Yamamoto1, Tsutomu Tamada2, Yu Ueda3, Takeshi Fukunaga2, and Atsushi Higaki2
1Radiology, Kawasaki Medical School, Kurashiki, Japan, 2Kawasaki Medical School, Kurashiki, Japan, 3Phillips Japan, Tokyo, Japan
This study suggests the possibility that
MRI using the values of T2 in cortex and T2* in medulla, which can sensitively
capture edematous changes in the renal cortex and hypoxia in renal medulla, can
be used to evaluate early diabetic nephropathy non-invasively and in a short
period of time.
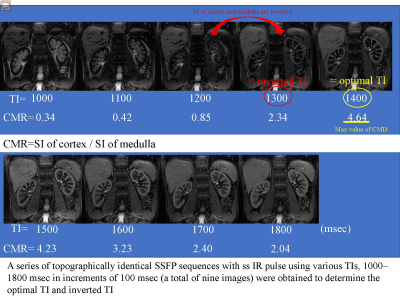
Recent studies of MR imaging have shown that water content level of tissue could be evaluated sensitively with T2 mapping 1). Steady-state free precession (SSFP) with spatially selective inversion recovery (ss IR) pulse using multi inversion time (TI) is able to distinct renal corticomedullary differentiation
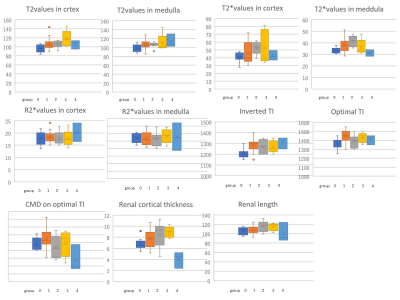
In
two-group comparisons, significant differences were seen between group 0 and
group 1 in values of T2 (mean ± SD: 95.8±8.39 vs. 106.4±11.49; p=0.015) T2* (33.4±2.41 vs. 38.3.0±6.31;
p=0.020), inverted TI (1205±49.4 vs. 1288±55.3; p=0.001) and optimal TI (1367±61.2
vs. 1450±60.0; p=0.002). Significant differences were seen between group 0 and group
2 in values of T2 (95.8±8.39 vs. 105.7±6.38; p=0.021) T2* (33.4±2.41 vs. 41.4±4.69;
p=0.001)
-
Quantitative T1 and R2* mapping in the evaluation of renal function in chronic kidney disease
Jiaxin Yan1, Weiqiang Dou2, Hongmei Gu1, Xinquan Wang1, Weiyin Vivian Liu2, Huijian Lu1, Ying Zhou1, Xuejun Zhou1, and LI Yuan1
1Affiliated Hospital of Nantong University, Nantong, China, 2GE Healthcare, MR Research China, Beijing, China
T1 mapping combined with BOLD-MRI derived R2* mapping might provide an effective method in assessing renal function.
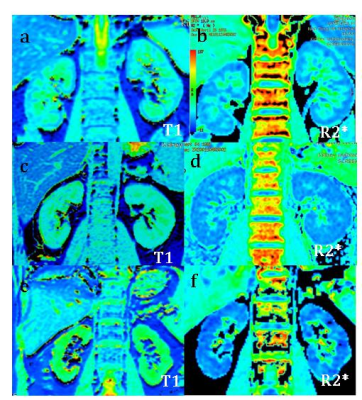
Fig.1
Representative T1 and
R2* mapping of the normal (a,b), the mild (c,d)
and the moderate to severe patients (e,f) were shown.
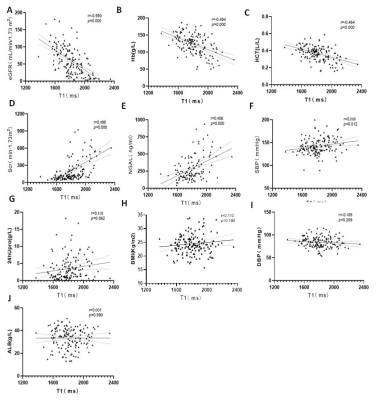
Fig. 3
A-C:T1 value was negatively correlated with
eGFR, Hb and HCT(p<0.05);
D-F:T1 value was
positively correlated with the NGAL, Scr, SBP(p<0.05); G-J: T1 value has no correlation with
24hUpro, BMI, DBP
and ALB.
-
Evaluation of renal oxygenation and hemodynamics in patients with chronic kidney disease by BOLD-MRI and intrarenal Doppler ultrasonography
Jing Yang 1, Shuohui Yang 2, Zheng He3, Mengxiao Liu4, and Caixia Fu5
1Nephrology, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China, 2Radiology, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China, 3Ultrasonography, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China, 4MR Scientific Marketing, Siemens Healthcare, Shanghai, China, 5MR Applications Development, Siemens Shenzhen Magnetic Resonance Ltd, Shenzhen, China
BOLD-MRI and IDU evaluating
renal oxygenation and hemodynamics on CKD
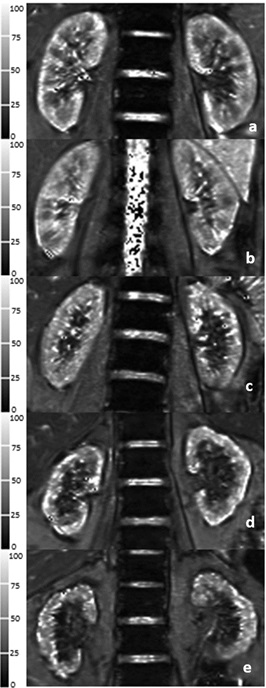
Fig.1 Five typical T2* images of BOLD-MRI in healthy
volunteer and CKD1-4 patients. a Healthy volunteer, male/26 years old. b
Stage 1 CKD patient, female/28 years old. c Stage 2 CKD patient, male/29
years old. d Stage 3 CKD patient, female/ 62 years old. e Stage 4
CKD patient, female/58 years old.
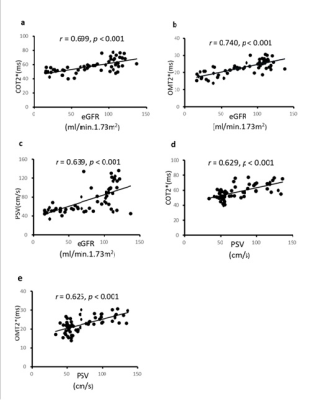
Fig.2 Correlation
coefficients among the cortical T2* (COT2*), outer
medullary T2* (OMT2*) values, peak systolic velocity (PSV), and
estimated glomerular filtration rate (eGFR). a Correlation
of COT2* values and eGFR. b Correlation of OMT2*
values and eGFR. c Correlation of PSV values and eGFR. d Correlation
of COT2* values and PSV
values. e Correlation of OMT2* values and PSV. Spearman coefficient
testing was performed to assess statistical significance.
-
Renal hypoxia estimated by O2-inhalation T2* BOLD MRI: association with renal dysfunction and left ventricular remodeling in cardiomyopathy
Michinobu Nagao1, Kiyoe Ando1, Yasuhiro Goto1, Isao Shiina1, Kazuo Kodaira1, Masami Yoneyama2, Takashi Namiki2, Atsushi Yamamoto1, Eri Watanabe1, Akiko Sakai1, Risako Nakao1, and Shuji Sakai1
1Tokyo Women's Medical University, Tokyo, Japan, 2Philips Japan, Tokyo, Japan
O2-inhalation T2*-BOLD MRI is a non-invasive imaging techniqueto evaluate renal oxygenation. Renal hypoxia expressed as decreased ΔR2*ratio is strongly associated with reduced eGFR. Early LV remodeling can lead to renal hypoxia.
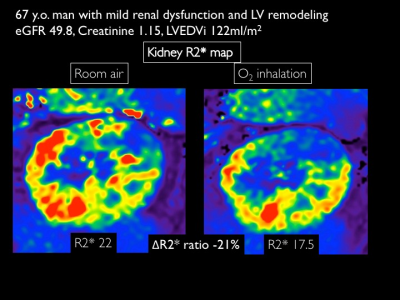
O2-inhalation T2* BOLD MRIR2* map for 67 years man with cardiomyopathy and mild renal dysfunction
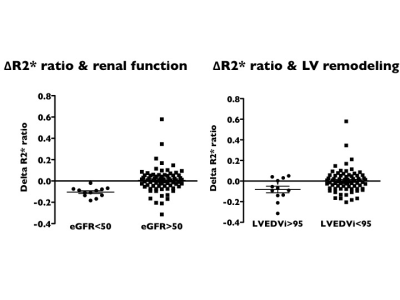
Comparison of ΔR2*ratio between patients with eGFR <50mL/min/1.73m2and >50 mL/min/1.73m2(left) and between patient with LVEDVi >95 ml/m2 and <95 ml/m2.
-
The value of intravoxel incoherent motion diffusion-weighted imaging and T1-Mapping in the evaluation of renal transplantation function
Dejuan Shan1,2, Xianquan Cui3, Xiangtao Lin1,2, Ruiyuan Diao2, Peng Zhao2, Mengxiao Liu4, Shuai Zhang2, Xiaoli Li2, Nan Lin2, Zhongyu Hou2, and Bing Liu5
1Department of Radiology, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, China, Jinan, China, 2Department of Radiology,Shandong Provincial Hospital Affiliated to Shandong First Medical Uiversity, Jinan, China, 3Qilu Hospital of Shandong University, Jinan, China, 4MR Scientific Marketing, Diagnostic Imaging, Siemens Healthcare Ltd., Shanghai 201318, China, Shanghai, China, 5Department of Radiology, China-Japan Friendship Hospital, Beijing, China, Beijing, China
T1 mapping shows a promising prospect in the evaluation of Renal allograft function, and can provide the early detection of impairment of the renal allograft function.
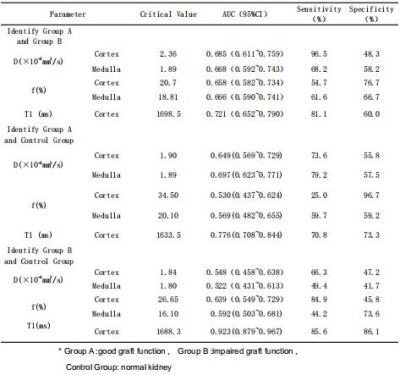
Table2. ROC Curve analysis of Renal allograft function in patients with Group A and Group B, Group B and Control group by IVIM and T1-Mapping
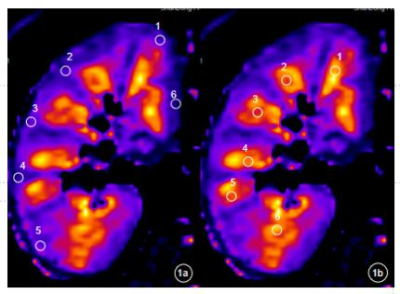
Fig. 1 The Kidney,s selection method of region of interest (ROI). 1a) the upper, middle and lower poles of the kidney are located in the kidney, and several ROIs are delineated in thecortex;1 b) the upper, middle and lower poles of the kidney are located in the kindey, and several ROIs are delineated in the Medulla
-
Native T1 mapping in assessment of kidney fibrosis in patients with chronic glomerulonephritis
Zhaoyu Shi1, Fangfang Shang1, Xinquan Wang1, Hongmei Gu1, Xiaoyan Liu1, Weiqiang Dou2, Weiyin Vivian Liu2, Yuan Zhang1, Jianhua Wu1, and Li Yuan1
1Affiliated Hospital of Nantong University, Nantong, China, 2GE Healthcare, MR Research China, Beijing, China
Native T1-mapping demonstrated good diagnostic
performance in evaluation of renal function and non-invasive detection of fibrosis in
chronic
glomerulonephritis patients.
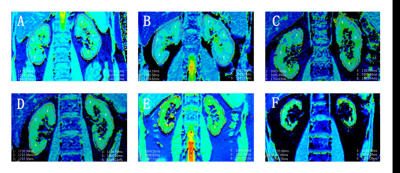
Fig.1
Renal T1 maps. A: Coronal native T1 map of two kidneys of a healthy subject,
T1=1539ms. B: CKD stage 1, T1=1622ms. C: CKD stage 2, T1=1751ms. D: CKD stage
3, T1=1796ms. E: CKD stage 4, T1=1902ms. F: CKD stage 5, T1=2068ms.
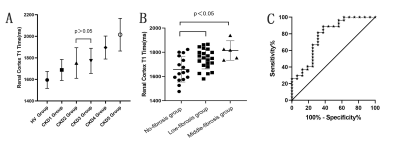
Fig.2 A: T1 value of renal cortex was significantly lower in
the HC group than in CKD patients(F=29.62, P<0.001). Statistical differences of
T1 values were found between CKD stages except for stage 2 and 3 (p<0.05).
B: Compared to non-fibrosis group, T1 value increased in the low and medium
fibrosis group (p<0.05). C: The area under the ROC curve of T1 value for the
prediction of IF is 0.762, and the optimal cutoff value of T1 is 1695 ms.
-
Evaluation of renal function in healthy volunteers and patients with chronic kidney disease by using APT weighted imaging and R2* mapping
Ye Ju1, Ailian Liu1, Jiazheng Wang2, Wenjun Hu1, Changyu Du3, Lingli Qi3, Haoyang Jiang3, Xinmiao Bu1, Nan Wang1, and Peng Sun2
1First Affiliated Hospital of Dalian Medical University, Dalian, China, 2Philips Healthcare, Beijing, China, 3Dalian Medical University, Dalian, China
The AUC, sensitivity and specificity of APT combined R2* were 0.925, 88% and 100%, respectively. The combination of APT and R2* can improve the diagnostic ability of discriminating CKD from HVs, which has certain clinical application value.
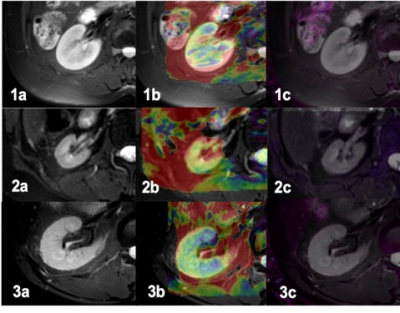
Figure 1. A 55-year-old female volunteer. T2w image (1a), APT image(1b), R2* image(1c). A 34-year-old female with CKD grade 5, eGFR was 3.96, T2w image (2a), APT image(2b), R2* image(2c). A 59-year-old male with CKD grade 1, eGFR was 114.58. T2w image (3a), APT image(3b), R2* image(3c).
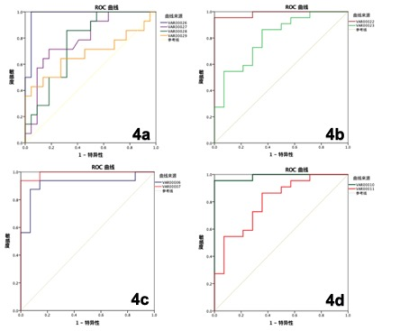
Figure 4. Diagnostic efficiency curve of VHs and mRD(4a), diagnostic efficiency curve of VHs and sRD(4b), diagnostic efficiency curve of the APT values in mRD and sRD(4c) , diagnostic efficiency curve of joint use of the APT values and the R2* value in VHs and mRD (4d).
