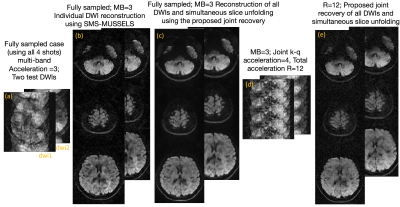
-
Improved Super-Resolution reconstruction for DWI using multi-contrast information
Xinyu Ye1, Pylypenko Dmytro1, Yuan Lian1, Yajing Zhang2, and Hua Guo1
1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 2MR Clinical Science, Philips Healthcare, Suzhou, China
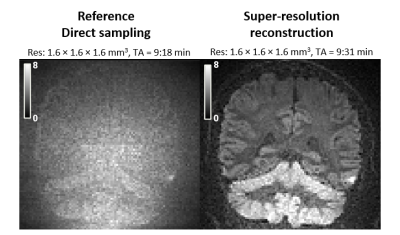
We propose an improved deep-learning based 3D super resolution
network to increase resolution for DWI images. With the help of anatomical
images and a novel FA loss function, the proposed method improves the
reconstruction accuracy.
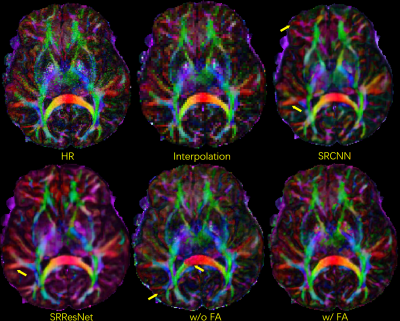
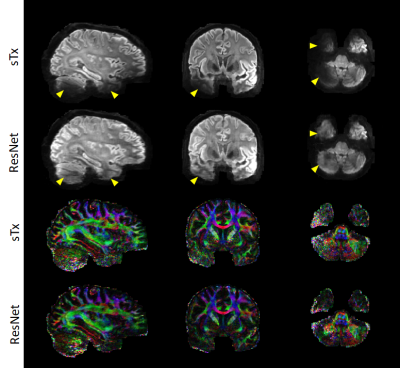
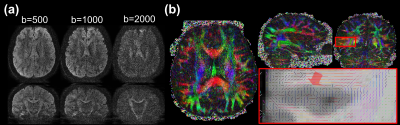
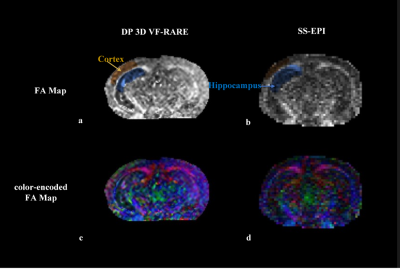
Fig. 5. Colored FA maps of different methods. The proposed method can recover fine fiber structures. With the introduction of FA loss function, the contrast contamination among diffusion directions can be reduced.
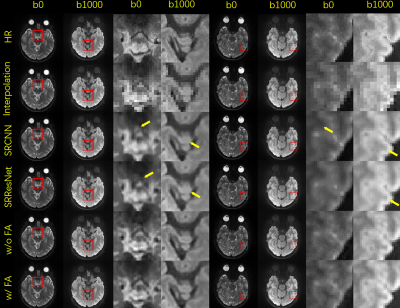
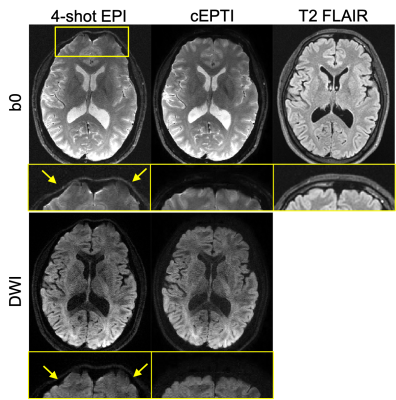
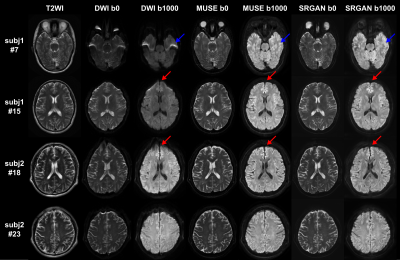
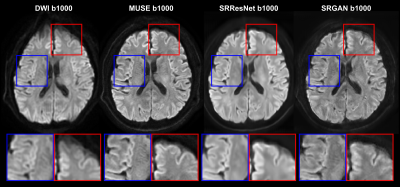
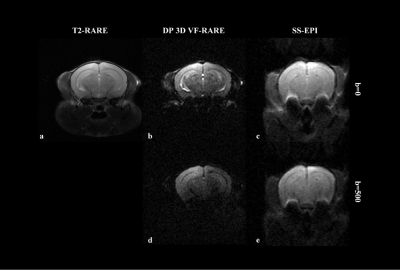
Fig. 3. Selected comparison results and zoomed-in images of in-vivo DWI data. b0 and mean DWI results from 2 representative slices are shown. In the zoomed-in images, the arrows point to the structures that SRCNN and SRResNet fail to recover.
-
A Model-driven Deep Learning Method Based on Sparse Coding to Accelerate IVIM Imaging in Fetal Brain
Tianshu Zheng1, Cong Sun2, Guangbin Wang2, Weihao Zheng1, Wen Shi1, Yi Sun3, Yi Zhang1, Chuyang Ye4, and Dan Wu1
1Key Laboratory for Biomedical Engineering of Ministry of Education, Department of Biomedical Engineering, College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, Zhejiang, China,, Zhengjiang University, Hangzhou, China, 2Department of Radiology, Shandong Medical Imaging Research Institute, Cheeloo College of Medicine, Shandong University, 324, Jingwu Road, Jinan, Shandong, 250021, People's Republic of China, Shandong University, Jinan, China, 3Department of Radiology 2MR Collaboration, Siemens Healthcare China, Shanghai, China, Siemens Healthcare China, Shanghai, China, 4chool of Information and Electronics, Beijing Institute of Technology, Beijing Institute of Technology, Beijing, China
We
proposed a Model-driven deep learning neural network based on sparse coding
which reliably predicted the IVIM parameters in the fetal brain with only a
subset of the diffusion data while retaining good interpretability, and
therefore, could potentially accelerate IVIM acquisition.
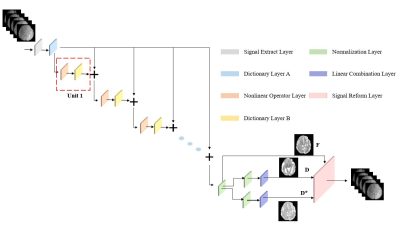
Figure1. Briefly, in the signal extract layer,
signals were extracted from each voxel as the input for
dictionary A. Each unit in the red dashed box
was comprised of a nonlinear operator layer and a dictionary
layer B. We chose ReLU
as the Nonlinear layer. This process was repeated 10 times. Then the output was transferred to the Normalization. [f, D, D*] can be obtained
using the linear combination layer (Eq [4-6]). Lastly, the output parameters
are fed into the signal reform layer according to IVIM model
to reconstruct the signals which are compared with the input as part of the loss function.
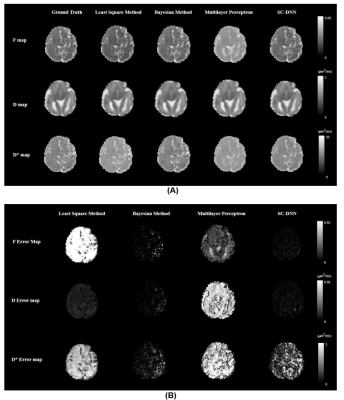
Figure2. (A) Estimated f, D, and D* parameter maps using the
least square or Baysian fitting of the bi-exponential model, multilayer
perceptron, and the proposed SC-DNN. The ground truth was obtained by adding
noise after recovering from the previous parameters according to the IVIM model.
(B) Residual error maps between the fitted parameters and
the ground truth via different methods.
-
Jointly Denoise Diffusion-weighted Images Using a Weighted Nuclear Norm Minimization Approach
Yujiao Zhao1,2, Linfang Xiao1,2, Zhe Zhang3, Yilong Liu1,2, Hua Guo4, and Ed X. Wu1,2
1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, China, 3China National Clinical Research Center for Neurological Diseases, Beijing Tiantan Hospital, Capital Medical University, Beijing, China, 4Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China
A
joint denoising method for diffusion-weighted images using low-rank matrix
approximation is proposed. It exploits structural similarities of DW images, leading
to significant noise reduction in all DW images and revealing more microstructural
details in quantitative diffusion maps.
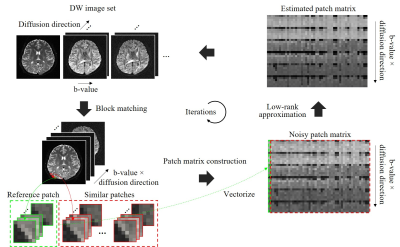
Fig. 1. Diagram of the proposed joint denoising method.
Within each iteration: (1) extracting reference patches using a sliding window
and searching for similar patches through block matching; (2) for each
reference patch, stretching
its similar patches to vectors and stacking them into a matrix to form a
low-rank patch matrix; (3) for
each patch matrix, estimating a noise-free patch matrix through a
weighted nuclear norm minimization (WNNM) model; (4) converting estimated patch
matrices back to images.
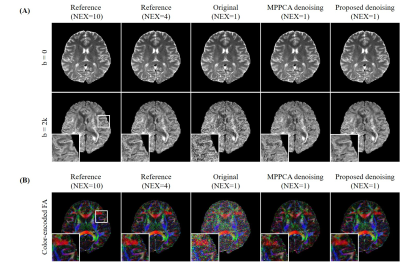
Fig. 3. Denoising results with in vivo DW brain images (A) and resulting
diffusion metric maps computed from denoised DW images (B). The image set contains one b0 image and 6 DW images with b =2000
s/mm2. Only DW image along one
direction is shown. The image set of NEX=1 was used for denoising, while
the image set of NEX=4/10 was used as high SNR reference. At very low SNR, the proposed method
was still robust and more effective than MPPCA in reducing noise while
recovering structural details when compared to reference. It achieved image
quality and FA map comparable to those using 4 averages.
-
SuperDTI: Superfast Deep-learned Diffusion Tensor Imaging
Hongyu Li1, Zifei Liang2, Chaoyi Zhang1, Ruiying Liu1, Jing Li3, Weihong Zhang3, Dong Liang4, Bowen Shen5, Peizhou Huang6, Sunil Kumar Gaire1, Xiaoliang Zhang6, Yulin Ge2, Jiangyang Zhang2, and Leslie Ying1,6
1Electrical Engineering, University at Buffalo, State University of New York, Buffalo, NY, United States, 2Center for Biomedical Imaging, Radiology, New York University School of Medicine, New York, NY, United States, 3Radiology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China, 4Paul C. Lauterbur Research Center for Biomedical Imaging, Medical AI research center, SIAT, CAS, Shenzhen, China, 5Computer Science, Virginia Tech, Blacksburg, VA, United States, 6Biomedical Engineering, University at Buffalo, State University of New York, Buffalo, NY, United States
This paper demonstrates
the feasibility of superfast DTI and fiber tractography
using deep learning with as few as six corrupted
DWIs (up to 30-fold). Such a significant reduction in scan time will allow
the inclusion of DTI into clinical routine for many potential applications.
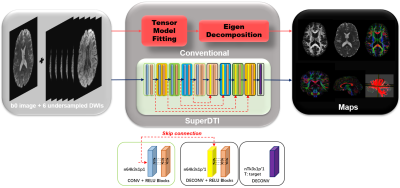
FIGURE
1. Schematic comparison of the conventional DTI model fitting and deep learning
methods SuperDTI for generating various diffusion quantification maps.
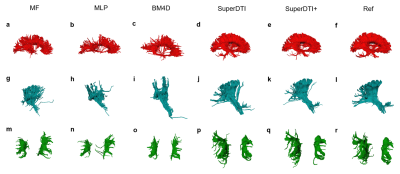
FIGURE 4. Comparison of fiber tractography generated from 6
DWIs using different methods. Corpus callosum, internal
capsule/corticospinal tract, and superior longitudinal fasciculus generated by
MF (a), MLP (b), BM4D (c), proposed SuperDTI (d), proposed SuperDTI+ (e) with
additional k-space reduction (l), respectively, and the corresponding difference
map (g-k) with 6 DWIs. The model-fitted tractography from 90 DWIs (d, k, r) is
also shown as a reference.
-
Deep learning for synthesizing high-b-value DWI of the prostate: A tentative study based on generative adversarial networks
lei hu1, jungong Zhao1,2, Caixia fu3, and Thomas Benkert4
1Department of Diagnostic and Interventional Radiology, Shanghai Jiao Tong University Affiliated Sixt, 上海, China, 2Department of Diagnostic and Interventional Radiology, Shanghai Jiao Tong University Affiliated Sixt, shanghai, China, 3MR Application Development, Siemens Shenzhen magnetic Resonance Ltd, shanghai, China, 4MR Application Predevelopment, Siemens Healthcare, Erlangen, Gernmany, Erlangen, Germany
A deep learning framework based on GAN is a promising
method to synthesize realistic high-b-value DWI sets with good image quality
and accuracy in PCa detection.
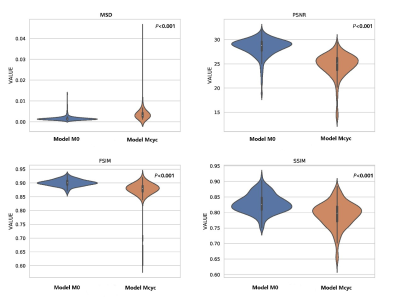
Fig.1 Violin plots of distributions of the
quantitative metrics between M0 and Mcyc.
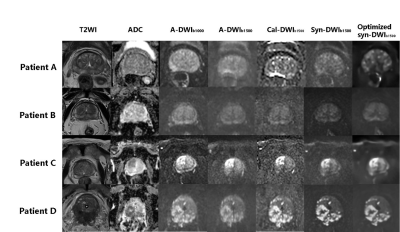
Fig.2 T2WI, ADC,
a-DWIb1000, a-DWIb1500, cal-DWIb1500, non-optimized
syn-DWIb1500 vs. optimized syn-DWIb1500 of four
different patients.
Patient A: A 76-year-old man with chronic prostatitis. Patient B: A 63-year-old man with prostatic hyperplasia. Patient C: An 87-year-old
man with prostatic cancer
in the right transition zone. (Gleason
score, 4+3). Patient D: A 69-year-old man with prostatic cancer
in the peripheral zone invading the rectum (Gleason score 4+5).
-
IVIM Imaging of Lung Cancer: A Comparison Between Gradient-and Spin-Echo, Turbo Spin-Echo and Echo-Planar Imaging Techniques
Tianyu Zhang1, Yishi Wang2, Chengxiu Yuan1, Xiaoyu Wang1, Jia Zhao1, and Huaqiang Sheng1
1The First Affiliated Hospital of Shandong First Medical University, Jinan, China, 2Philips Healthcare, Beijing, China
Single-shot gradient- and spin-echo (SS-GRASE) technology is a useful technique for IVIM imaging of lung cancer
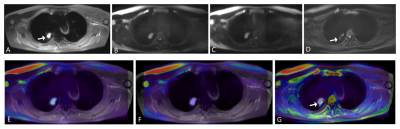
FIGURE 1. A nodular lesion (arrow) in the right upper lobe. A, T2WI-SPIR. B, GRASE-IVIM (b = 800 s/mm2 ). C, TSE-IVIM (b = 800 s/mm2 ). D, EPI-IVIM (b = 800 s/mm2 ).E,T2WI fused with GRASE-IVIM. F, T2WI fused with TSE-IVIM.G, T2WI fused with EPI-IVIM. The distortion and displacement of the lesion are shown on EPI-IVIM (arrow). On TSE and GRASE-IVIM ,the lesion, along with its air bronchus, can be clearly shown. Fused images showed that GRASE and TSE-IVIM is perfectly matched with T2WI-SPIR.
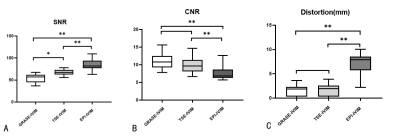
FIGURE 2. Comparison of SNR,CNR and Distortion between GRASE-IVIM,TSE-IVIM and EPI-IVIM.
-
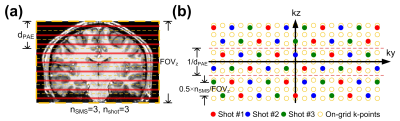
Flip-angle optimization for the diffusion-weighted SPLICE sequence for applications in brain imaging
Sofie Rahbek1, Tim Schakel2, Faisal Mahmood3,4, Kristoffer H. Madsen5,6, Marielle E.P. Philippens2, and Lars G. Hanson1,5
1Department of Health Technology, Technical University of Denmark, Kgs. Lyngby, Denmark, 2Department of Radiotherapy, University Medical Center Utrecht, Utrecht, Netherlands, 3Laboratory of Radiation Physics, Odense University Hospital, Odense, Denmark, 4Department of Clinical Research, University of Southern Denmark, Odense, Denmark, 5Danish Research Centre for Magnetic Resonance, Centre for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital Hvidovre, Hvidovre, Denmark, 6Department of Applied Mathematics and Computer Science, Technical University of Denmark, Kgs. Lyngby, Denmark
The diffusion-weighted SPLICE sequence was improved using the proposed framework for optimization of individual refocusing flip-angles in the echo-train. This provides optimal SNR for a given spatial point spread function representing a desired resolution and acceptable Gibbs ringing.
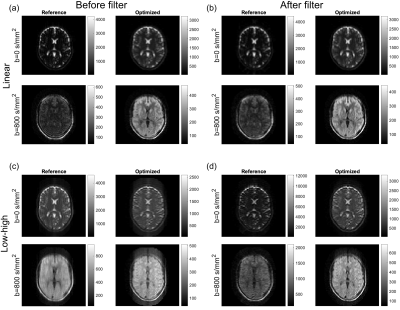
Brain images for the reference scheme (constant FA of 120°) and optimized FA scheme, respectively. (a-b): Data sampled with linear phase-encoding order before and after applying calculated filters. (c-d): Data sampled with center-out phase-encoding order before and after applying calculated filters.

The Hanning target function (a) and corresponding PSF (b) having a low FWHM and limited ripples. The optimized FAs (c) together with the reference scheme of 120° flips. The first four echoes are marked as “transients” and discarded for improved robustness. The resulting filters for both echo families, E1 and E2, normalized with the coefficient used for the center of k-space.
-
Validating the Accuracy of Multi-Spectral Metal Artifact Suppressed Diffusion-Weighted Imaging
John Neri1, Matthew F Koff1, Kevin M Koch2, and Ek Tan1
1Radiology and Imaging, Hospital for Special Surgery, New York, NY, United States, 2Medical College of Wisconsin, Wauwatosa, WI, United States
MAVRIC diffusion weighted imaging can image tissue microstructure in
peri-prosthetic regions with strong magnetic susceptibility effects. This work
compares the accuracy of DWI-MAVRIC relative to conventional echo
planar-imaging-based DWI.
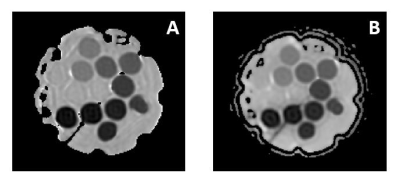
Figure 1: (A) Coronal DWI-EPI (NEX = 1) and (B)
Coronal DWI-MAVRIC (bins=3) images of 13 PVP vials contained in phantom. (A)DWI-EPI
contains low EPI distortion while (B) DWI-MAVRIC contains no EPI distortion.
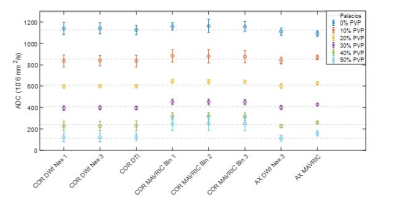
Figure 2: ADC mean values for eight acquired scans (six coronal, two
axial) at all six polymer concentrations. The dashed lines show the references
values from Palacios et al.(10) for all
six concentrations.
-
Quantitative Accuracy of Diffusion-Weighted Imaging Techniques as a Function of Susceptibility Artifact Resilience
Volkan Emre Arpinar1,2, Alexander D Cohen1, Sampada Bhave3, and Kevin M Koch1,2
1Radiology, Medical College of Wisconsin, Milwaukee, WI, United States, 2Center for Imaging Research, Medical College of Wisconsin, Milwaukee, WI, United States, 3Canon Medical Research USA, Cleveland, OH, United States
There are several different acquisition approaches to mitigate susceptibility artifacts in DWI. The quantification performance of a selection of these approaches were evaluated in phantom and volunteer experiments.
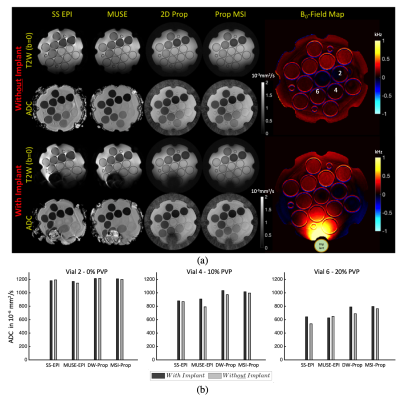
Fig.3. (a) Representative T2W(b=0) images and associated ADC maps: ADC's are shown for each of the four evaluated methods. Significant distortion seen in the SS-EPI, both with and without the presence of the metal high susceptibility source. Without the metal, image distortions were relatively mitigated with MUSE EPI. Qualitatively, DW-Prop and MSI-Prop were less susceptible to susceptibility artifacts. Vial 6 was largely non-viable in the SS-EPI and MUSE-EPI, but showed perfect geometry in DW & MSI-Prop. Vial 6 were compromised in DW-Prop, but were fully recovered in MSI-Prop.
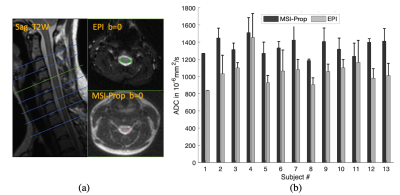
Fig.4. (a) Representative EPI and MSI-Prop T2W (b=0) images and spinal cord ROIs. Significant distortions can be seen for the EPI. MSI-Prop provided more anatomically accurate imaging of the cord. (b) ADC values computed with MSI-Prop were roughly 20% larger on average than the FOCUS EPI images. The computed bias was 293·10-6mm2/s among 13 subjects’ mean cord ADC values.
-
Simultaneous Reconstruction of High-resolution Multi b-value DWI with Single-shot Acquisition
Fanwen Wang1, Hui Zhang1, Fei Dai1, Weibo Chen2, Chengyan Wang3, and He Wang1,3
1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 2Philips Healthcare, Shanghai, China, 3Human Phenome Institute, Fudan University, Shanghai, China
This study proposed a novel
method to reconstruct four-shot high-resolution DWIs from one-shot data for
multiple b-values simultaneously, enabling the physiological feature
transformation through different b-value.
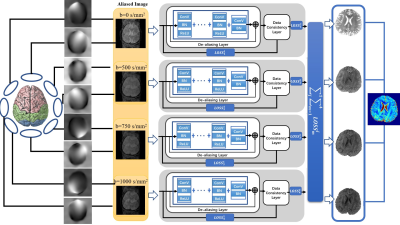
Figure 1: The proposed model for multi-coil
high-resolution DWI reconstruction. The network combines losses from four channels
to back propagate. Each channel consists of a de-aliasing layer and a data consistency
layer to recover from aliasing artifacts.
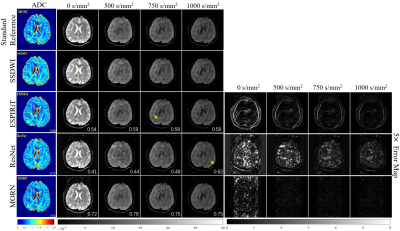
Figure 2: Comparison of
reconstructed images for multiple b-values in healthy volunteers. The
calculated ADC maps are shown leftmost. The b-values are listed on each column.
SSIMs are listed at the bottom-right of each image. The rightmost four columns
represent the corresponding error maps. The error was x5 amplified for better
visualization. The aliasing artifacts are shown by arrows.
-
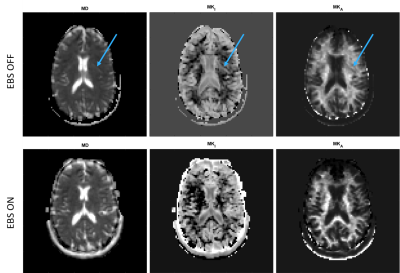
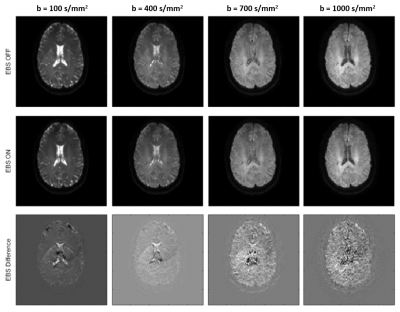
Robust method for Whole Body DWIBS applied both Image based B0 Shimming and Blip-up Blip-down Distortion Correction
Hiroshi Hamano1, Masami Yoneyama1, Yasutomo Katsumata2, Kazuhiro Katahira3, and Kenji Iinuma1
1Philips Japan, Tokyo, Japan, 2Philips Healthcare, Tokyo, Japan, 3Department of Radiology, Kumamoto Chuo Hospital, Kumamoto, Japan
DWIBS sometimes suffers from sever
image distortion due to the presence of air within and/or at edge of the FOV.
We demonstrated that whole body DWIBS applied both image based B0 shimming and
blip-up blip-down distortion correction to provide higher robustness.
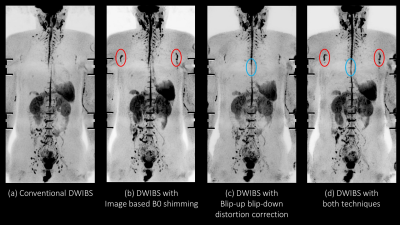
Figure 2. The MIP of direct
coronal whole body DWIBS at 3.0T were shown. Image based B0 shimming provided
improving depiction of axillary lymph nodes (red circle). Blip-up blip down
distortion correction also decrease distortion effect due to the presence of
air (blue circle).
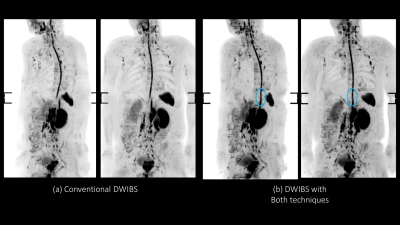
Figure 4. The MIP of transverse DWIBS at 1.5T were shown. Similar findings
to 3.0T were obtained at 1.5T.
-
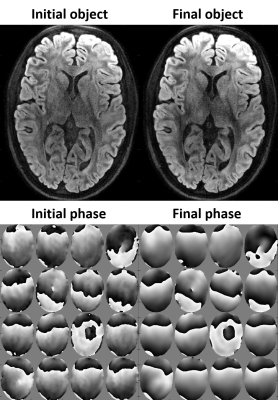
Is Perfect Filtering Enough Leading to Perfect Phase Correction?
Feihong Liu1,2, Junwei Yang2,3, Zhiming Cui2,4, Xiaowei He1,5, Jun Feng1,5, and Dinggang Shen2,6,7
1School of Information Science and Technology, Northwest University, Xi'an, China, 2School of Biomedical Engineering, ShanghaiTech University, Shanghai, China, 3Department of Computer Science and Technology, University of Cambridge, Cambridge, United Kingdom, 4Department of Computer Science, The University of Hong Kong, Hong Kong, China, 5State-Province Joint Engineering and Research Center of Advanced Networking and Intelligent Information Services, Xi'an, China, 6Shanghai United Imaging Intelligence Co., Ltd., Shanghai, China, 7Department of Artificial Intelligence, Korea University, Seoul, Korea, Republic of
We calibrated the phase correction procedures with the goal of unbiased white matter microstructure estimation.
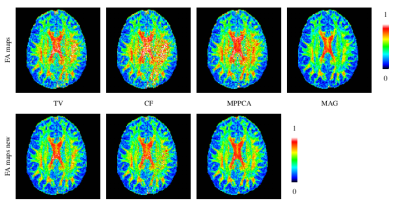
`FA maps new' denotes the phase correction results obtained by the renewed procedures. Artifacts are significantly eliminated for all three methods, while the FA values in corpus callosum is increased properly.

`TV new', `CF new', and `MPPCA new' denote the phase correction results obtained by the renewed procedures. The renewed procedures yield more accurate FA values, especially for MPPCA. Black color of white matter means high accuracy achieved. MAG denotes the magnitude images.
-
Optimal Diffusion Sampling Scheme for High Performance Gradients
Nastaren Abad1, Luca Marinelli1, Radhika Madhavan1, James Kevin DeMarco2, Robert Y Shih2,3, Vincent B Ho2,3, Gail Kohls2, and Tom K.F Foo1
1General Electric Global Research, Niskayuna, NY, United States, 2Walter Reed National Military Medical Center, Bethesda, MD, United States, 3Uniformed Services University of the Health Sciences, Bethesda, MD, United States
To establish a benchmark for future studies this study utilized a data
driven approach for optimizing diffusion sampling for high-performance
gradients, focusing on b-value and noise performance on uncertainty in tensor estimates & fiber orientation to resolve sub-voxel information.
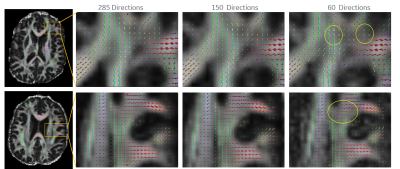
Figure
5. fODFs over two slices highlight exemplary insets as # directions is uniformly
decreased. Interestingly, at half the sample size of the superset, the
principal component is retained. Even with the sample size scaled to a 1/4th
of the original dataset, the principal component is retained, though, a slight
uptick in noise is evident in the fiber crossing and interfacial regions. (“noisy”
lobes: yellow circles).
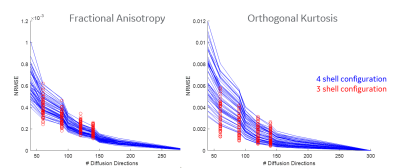
Figure
4. Normalized root mean square error (NRMSE) over 76 WM bundles for FA and
Orthogonal Kurtosis highlighting bias developed as # directions sampled is decreased. As
is evident, the NRMSE for both FA and kurtosis grows more slowly, indicating
stability compared to the standard, however, past 90 directions, the sqrt(N)
scaling factor breaks down for both measures albeit the bias is not at the same
scale. Interestingly, with uniform sampling over a 3-shell configuration, the
bias in metrics can be reduced. Note the y-axis scale for FA and Kurtosis is
not the same
-
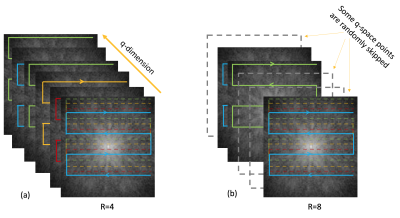
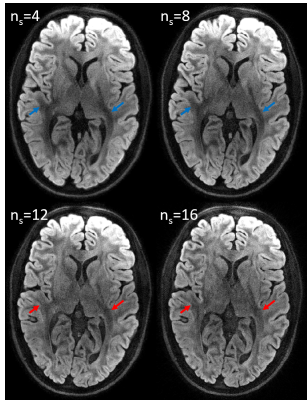
Highly-Accelerated Multi-shot Diffusion Imaging with High Angular Resolution Enabled by k-d SVD
Shihui Chen1 and Hing-Chiu Chang1
1Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong, Hong Kong
The reconstruction scheme
based on k-d SVD for high angular resolution diffusion imaging (HARDI) can provide images with improved quality at a
high in-plane acceleration factor without the limitation by the number of
coils.
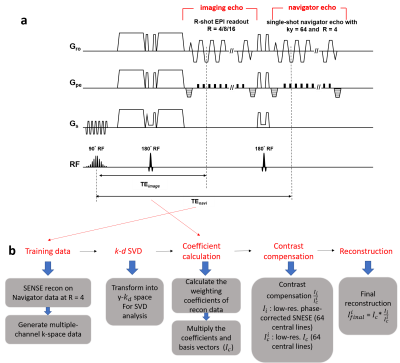
Figure 1 (a) The proposed design of interleaved DW-EPI with
navigator echo. The acceleration factor R of imaging can be 4, 8, or 16, while
R for the navigator echo is fixed at 4. (b) The reconstruction pipeline based
on proposed k-d SVD with contrast compensation at final step.
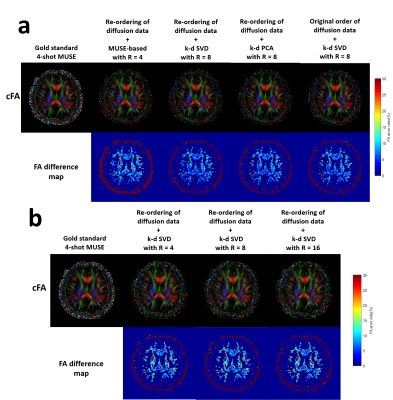
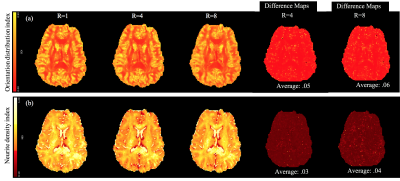
Figure 5 (a)The comparison of colour FA map derived from
gold standard images and the images reconstructed with the different strategies
in Simulation 1. (b) The comparison of colour FA map derived from gold standard
images and our proposed k-d SVD method at different acceleration factors (R =
4/8/16).
-
Improved multi-shot EPI ghost correction for high gradient strength diffusion MRI using Structured Low-Rank Modeling k-space reconstruction
Gabriel Ramos-Llordén1, Rodrigo A. Lobos2, Tae Hyung Kim1, Qiyuan Tian1, Slimane Tounetki1, Thomas Witzel3, Boris Keil4, Anatasia Yendiki1, Berkin Bilgic1,5, Justin P. Haldar2, and Susie Huang1,5
1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Masachusetts General Hospital, Harvard Medical School, Charlestown, MA, United States, 2Ming Hsieh Department of Electrical and Computer Engineering, University of Southern California, Los Angeles, CA, United States, 3Q Bio Inc, San Carlos, CA, United States, 4Institute of Medical Physics and Radiation Protection, Mittelhessen University of Applied Sciences, Giessen, Germany, 5Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States
Structured Low Rank matrix k-space reconstruction can significantly reduce ghosting artifacts in 3D multi-shot EPI diffusion MRI with high diffusion-encoding gradients, which otherwise persist with conventional reconstruction techniques.
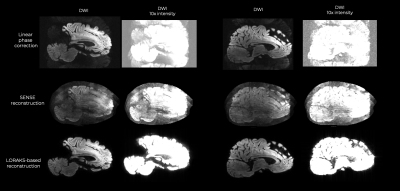
Two representative sagittal slices reconstructed with linear phase correction, SENSE, and LORAKS-based reconstruction. Note the marked ghosting reduction obtained with LORAKS-based k-space reconstruction.
-
Quantitative Evaluation of Multiband Diffusion MRI Data
Arun Venkataraman1, Benjamin Risk2, Deqian Qiu3,4, Jianhui Zhong1,5, Feng (Vankee) Lin6,7, and Zhengwu Zhang8
1Physics and Astronomy, University of Rochester, Rochester, NY, United States, 2Biostatistics and Bioinformatics, Emory University, Atlanta, GA, United States, 3Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, GA, United States, 4Biomedical Engineering, Emory University, Atlanta, GA, United States, 5Imaging Sciences, University of Rochester Medical Center, Rochester, NY, United States, 6Brain and Cognitive Sciences, University of Rochester, Rochester, NY, United States, 7Neuroscience, University of Rochester Medical Center, Rochester, NY, United States, 8Biostatistics and Computational Biology, University of Rochester Medical Center, Rochester, NY, United States
In this study, we quantified the effects of simultaneous multislice and phase acceleration on dMRI data quality, and how patient factors such as motion influenced data quality. Faster sequences showed less patient motion, but had a drop in signal-to-noise ratio and contrast-to-noise ratio.
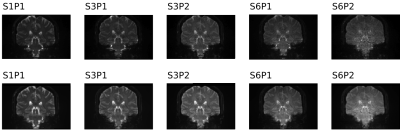
Figure 2 Representative section from Figure 1 normalized to highest intensity of each acquisition. The noise amplification in the midbrain and brain stem is easily visualized here (right-most sections in each row).
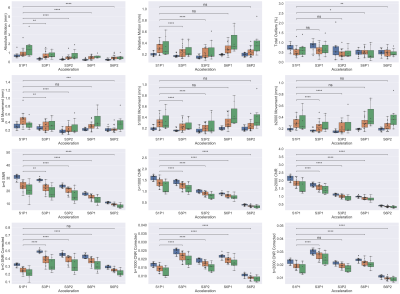
Figure 3 Boxplots representing quality control metrics (described on y-axis labels) over different acceleration schemes (x-axis). Blue bars indicate young subjects, orange indicates healthy, old subjects, and green indicates MCI subjects. p-values calculated using a Generalized Estimating Equation (GEE) with Gaussian assumption and exchangeable correlation accounting for repeated subject scans. p-values reported after false discovery rate (FDR) correction; significance: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
-
Deep Learning Based Super-resolution of Diffusion MRI Data
Zifei Liang1 and Jiangyang Zhang1
1Center for Biomedical Imaging, Dept. of Radiology, New York University School of Medicine, NEW YORK, NY, United States
We applied the hybrid-contrast images training to achieve the diffusion weighted images super-resolution. However, verified that distinct resolution or contrast sacrifice some efficacy of the super-resolution algorithm.
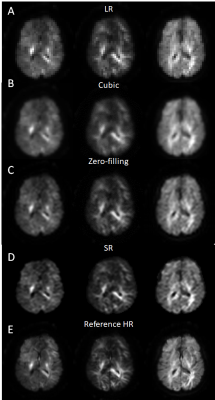
Figure 4. Super-resolution of human brain diffusion weighted images. From
top to the bottom row: A. initial Low resolution data; B. cubic interpolation;
C. Zero-filling interpolation; D. ResNet output by zero-filling as pre-step
interpolation; E. High resolution Reference).
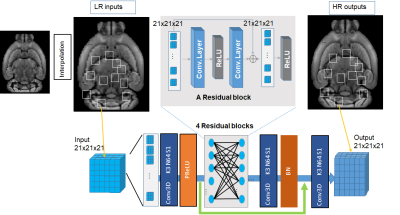
Figure 1. The architecture of ResNet architecture (input patch size
could be three dimensional 21x21x21 (or m×n×k) or two dimensional m×n, and
convolution kernel should be corresponded as three dimensional and two
dimensional).
-
A General Framework for Automated and Accurate b-Matrix Calculation for dMRI Pulse Sequences
Lisha Yuan1, Qing Li2, Guojing Wei3, Hongjian He1, and Jianhui Zhong4
1Department of Biomedical Engineering, Center for Brain Imaging Science and Technology, Zhejiang University, Hangzhou, China, 2MR Collaborations, Siemens Healthcare Ltd., Shanghai, China, 3SHS DI MR R&D SZN LP, Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China, 4Department of Imaging Sciences, University of Rochester, Rochester, NY, United States
A
general framework was proposed to calculate b-matrix automatedly and
accurately. Taking the effects of all gradient pulses into account, it provides
accurate b-matrix and is of great significance when the imaging gradients have
a non-negligible diffusion-related signal attenuation.
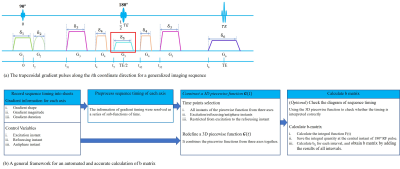
Figure 1. (a) A generalized imaging sequence along one coordinate direction, and (b) a general framework for an automated and
accurate calculation of b matrix.
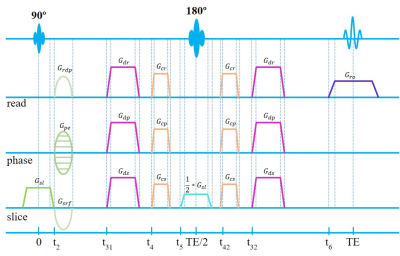
Figure
2. 2D SE imaging sequence
-
Automatic Phase Image Texture Analysis for Motion Detection in Diffusion MRI (APITA-MDD) with Adaptive Thresholding
Xiao Liang1, Pan Su2, Steve Roys1, Rao P Gullapalli1, Jerry L Prince3, and Jiachen Zhuo1
1Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 2Siemens Medical Solutions USA Inc, Malvern, PA, United States, 3Department of Electrical and Computer Engineering, Johns Hopkins University, Baltimore, MD, United States
We developed a more robust phase image-based motion detection method (APITA-MDD) that automatically generates ROI for motion detection and detects motion based on a robust metric . The APITA-MDD correctly identified motion at edge slices and motion caused by head rotation and leg crossing.
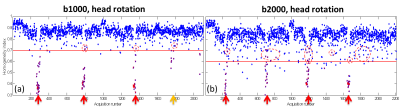
Figure 3. Scattered plots of HHI values for all slices for
an example b1000 (a) and b2000 (b) cases. Motion detected by PITA-MDD are all
dots fall below the HHI threshold, defined as 0.7 for b1000 and 0.6 for b2000
dMRI (red lines). Motion detected by APITA-MDD are denoted in red. Notice
slices missed by PITA-MDD but detected with adaptive thresholding in APITA-MDD
(circle). Arrows indicate time when motion instruction was given.

Figure 4. Heat maps of HHI (a) and the deviation score M (b) for
representative b1000 and b2000 acquisitions with no motion, head rotation, and
leg crossing motions. In each map, the horizontal axis represents volume index,
and vertical axis represent slices index. Range for HHI is [0,1]. Range for
deviation score is from 0 to maximum value 25.
-
Diffusion-Weighted Imaging with Integrated Slice-Specific Dynamic-Shimming for Rectal Cancer Detection and Characterization
Jianxing Qiu1, Jing Liu1, Chenwen Liu2, Jinxia Zhu2, and Thomas Benkert3
1Peking University First Hospital, Beijing, China, 2MR Collaboration, Siemens Healthcare Ltd China, Beijing, China, 3MR Application Development, Siemens Healthcare GmbH, Erlangen, Germany
DWI with iShim improves image quality of rectal cancer and help pathological differentiation.
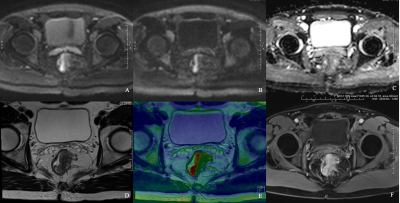
Figure 4 iShim DWI for patient with primary rectal cancer of Grade 1
DW images of b800 (A), b1600 (B), ADC map (C), T2WI (D), infusion images of both T2WI and DWI (E) and dynamic T1WI (F) showed the same location of lesion. Average ADC value measured was 1025.39 mm2/s (C).
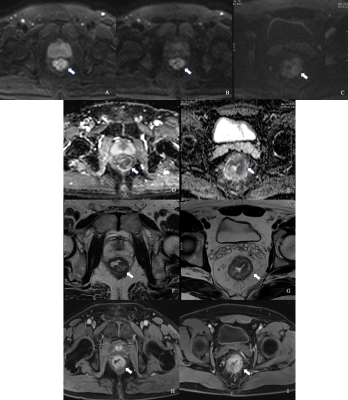
Figure 2 Comparison between iShim- and SS-EPI-DWI in patients after CRT.
Images on the left side were on the same location in patients with iShim-DWI. Images on the right side were on the same location in patients with SS-EPI-DWI. iShim-DWI of b800 (A) and b1600 (B) showed higher SNR and CNR with lower signal noise compared with SS-EPI-DWI of b1000 (C). ADC map (E) of iShim-DWI showed better image quality compared with ADC map (F) of SS-EPI-DWI. T2W images (F and G) and dynamic T1W images (H and I) showed the same location of lesion (arrows) for iShim- and SS-EPI- cohort, respectively.