-
Brain Parenchymal Venous System Plays a Substantial Role in Cerebral Waste Clearance
Yongsheng Chen1, Jiani Hu2, Yimin Shen2, Lara M Fahmy3,4, Li Zhang3, E. Mark Haacke1,2, and Quan Jiang1,3
1Department of Neurology, Wayne State University School of Medicine, Detroit, MI, United States, 2Department of Radiology, Wayne State University School of Medicine, Detroit, MI, United States, 3Department of Neurology, Henry Ford Health System, Detroit, MI, United States, 4Department of Psychiatry and Behavioral Neurosciences, Wayne State University School of Medicine, Detroit, MI, United States
We observed substantial
parenchymal venous participation in cerebral waste clearance in addition to
established cerebrospinal fluid participation.
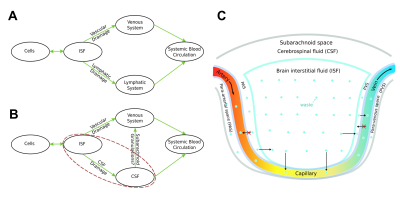
Figure 5. Schemes of waste
clearance. A: Schematic of waste clearance in body tissue outside the
brain. B: Schematic of CWC illustrating the participation of both the
venous system and CSF system; The main difference between CWC and body waste
clearance is the extra layer of CSF (red dashed line in B). C:
Illustration of the one-way transfer of cerebral waster from the brain
parenchyma to capillaries or venules as well as much smaller diameter of the
para-venous pathway comparing to the corresponding venous pathway, which makes
it less effective in waste clearance anatomically.
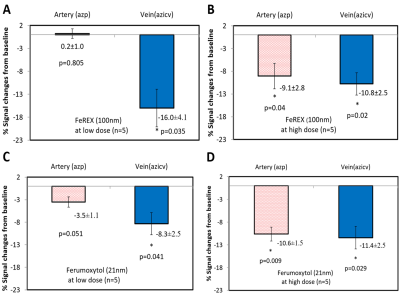
Figure 4.
Participation of the parenchymal venous pathway in CWC. SWI signal intensity
changes post-SPIO CSF tracer infusion in normal rats. A-B: Quantitative
MRI signal intensity changes pre-/post-low dose 75μg (A) and high dose
270 μg (B) FeREX (100nm) in azp and azicv; demonstrating CSF tracer
presence in azicv, but not azp, at low dose. C-D: Quantitative MRI
signal intensity changes pre-/post-low dose 75μg (C) and high dose 240
μg (D) Ferumoxytol (21nm) in azp and azicv; demonstrating CSF tracer
presence in azicv, but not azp, at low dose.
-
An investigation of the change in water diffusivity along the perivascular space in hypertensive patients
Junko Kikuta1, Koji Kamagata1, Kaito Takabayashi1, Toshiaki Taoka2, Hajime Yokota3, Yuki Someya4, Yoshifumi Tamura4,5, Hirotaka Watada4,5, Ryuzo Kawamori4,5, Shinji Naganawa6, and Shigeki Aoki1
1Department of Radiology, Juntendo University School of Medicine, Bunkyo-ku, Japan, 2Department of Innovative Biomedical Visualization, Graduate School of Medicine, Nagoya University, Nagoya, Japan, 3Department of Diagnostic Radiology and Radiation Oncology, Graduate School of Medicine, Chiba University, Chiba, Japan, 4Sportology Center, Juntendo University School of Medicine, Bunkyo-ku, Japan, 5Department of Metabolism & Endocrinology, Juntendo University School of Medicine, Bunkyo-ku, Japan, 6Department of Radiology, Nagoya University Graduate School of Medicine, Nagoya, Japan
We used the analysis along the perivascular space (ALPS) index, which has been
suggested as a noninvasive method to
measure water diffusivity along the perivascular space in vivo. We assessed the change in water diffusivity in living
patients with hypertension for the first time.
Fig.2. Region of interest (ROI) placement for the manual-based ALPS index
A 5-mm-diameter spherical ROI
was placed in the projection and association areas.
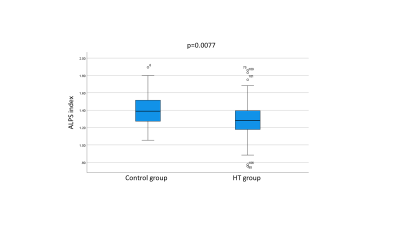
Fig.3. The Box plot of the ALPS index
The ALPS
index in the HT group was significantly lower than that in the control group (p
value < 0.05).
-
Assessment of cerebral white matter hemodynamics across the adult lifespan
Meher R. Juttukonda1,2, Randa Almaktoum1, Kimberly A. Stephens1, Kathryn Yochim1, Essa Yacoub3, Randy L. Buckner4, and David H. Salat1,2
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Radiology, Harvard Medical School, Boston, MA, United States, 3Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 4Psychology, Harvard University, Cambridge, MA, United States
We propose a novel approach for measuring white
matter ATTs from multi-PLD ASL data. Using this approach, we demonstrated that
CBF is lower (and decreases with age) and ATT is longer (and elongates with age)
in white matter compared to gray matter.
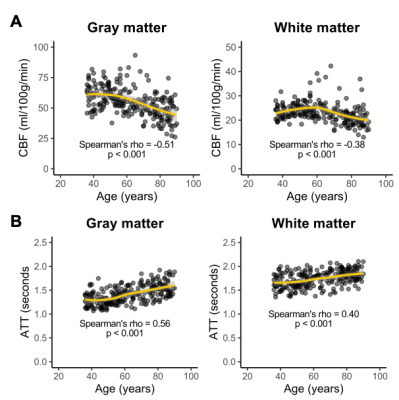
Figure 2: (A) CBF is, overall, inversely associated with age
in both gray matter and white matter. (B) ATT is directly associated with age in both gray
matter and white matter. However, these
relationships may be different when comparing early to late middle-age adults
to older adults.
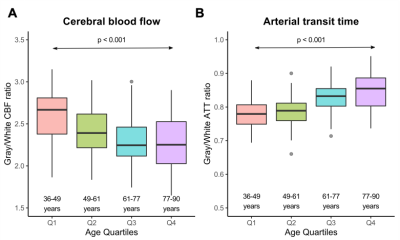
Figure 3: (A) The ratio of gray-to-white matter CBF generally
decreases with age until the fourth quartile in which there is more
variability. (B) The ratio of gray-to-white matter ATT also increases with age
and also exhibits greater variability in the fourth quartile.
-
High resolution imaging of choroid plexus blood flow with multi-delay pseudo-continuous arterial spin labeling
Xingfeng Shao1, Chenyang Zhao1, Matthew Borzage2, Catherine Mark3, Elizabeth Joe4, Jonathan Russin3,5, Charles Liu3,4,5, Darrin Lee3,5,6, and Danny JJ Wang1,4
1Laboratory of FMRI Technology (LOFT), Mark & Mary Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 2Fetal and Neonatal Institute, Division of Neonatology, Children's Hospital Los Angeles, Department of Pediatrics, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 3Department of Neurological Surgery, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 4Department of Neurology, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 5USC Neurorestoration Center, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 6Department of Psychiatry, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States
An high spatiotemporal resolution ASL sequence and a denoising method were developed to measure dynamic choroid plexus (CP) perfusion. CP blood flow was altered in normal pressure hydrocephalus patient and has a potential relation with water exchange rate across the blood-brain barrier.
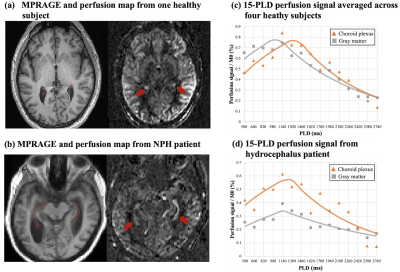
Figure 3. MPRAGE and perfusion map from one healthy subject (a) and NPH patient (b). CP was indicated by red arrows. Enlarged ventricle as well as choroid plexus hyperplasia can be observed in the NPH patient. (c) and (d) show scatter plot of average perfusion signal and fitted curves in GM and CP averaged across four healthy subjects and in the NPH patient, respectively. A delayed peak with comparable signal intensity was observed in CP as compared to GM in healthy subjects, while overall CP flow signal was relatively higher than GM in the NPH patient.
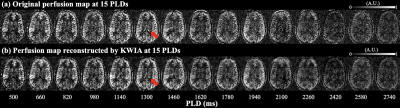
Figure 2. A center slice of perfusion image acquired at fifteen PLDs through direct subtraction (a) and KWIA filtering (b). CP was indicated by red arrows.
-
ADC change during cardiac cycle in idiopathic normal pressure hydrocephalus before and after tap test and shunt surgery
Ryo Yagawa1, Naoki Ohno1, Tosiaki Miyati1, Mitsuhito Mase2, Tomoshi Osawa2, Harumasa Kasai2, Yuta Shibamoto2, and Satoshi Kobayashi1
1Division of Health Sciences, Kanazawa University, Kanazawa, Japan, 2Nagoya City University Hospital, Nagoya, Japan
The frontal white matter ΔADC in iNPH decreased after the lumbar tap
and shunt surgery. ΔADC analysis may provide detailed information regarding
changes in the hydrodynamic and biomechanical properties through CSF drainage.
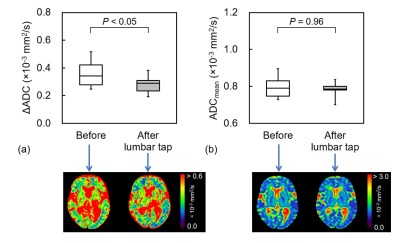
Figure 1. (a) ΔADC and (b) ADCmean in the
positive group before and after the lumbar tap, and each of representative
images.
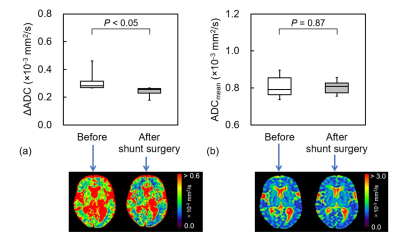
Figure 3. (a) ΔADC and (b) ADCmean in the
positive group before and after the shunt surgery, and each of representative
images.
-
Long term evaluation of ventricular volume change associated with shunt-responsiveness in idiopathic normal pressure hydrocephalus
Romtheera Kamronritthisorn1, Kritdipha Ningunha2, Peeratat Suppapanya1, Sunee Bovonsunthonchai3, Doonyaporn Wongsawaeng 1, Yudthaphon Vichianin4, Theerapol Witthiwej5, Weerasak Muangpaisan6, Panida Charnchaowanish1, Siriwan Piyapittayanan1, Orasa Chawalparit1, and Chanon Ngamsombat1
1Department of Radiology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand, 2Department of TELE-Radiology, Bangkok Hospital Headquarters, Bangkok, Thailand, 3Faculty of Physical Therapy, Mahidol University, Bangkok, Thailand, 4Department of Radiological Technology, Faculty of Medical Technology, Mahidol University, Bangkok, Thailand, 5Division of Neurosurgery, Department of Surgery, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand, 6Department of Preventive and Social Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
A significant reduction of ventricular volume after shunt surgery in idiopathic normal pressure hydrocephalus patients is found among long term shunt responders andhas a negative correlation with improved cognitive clinical scores.
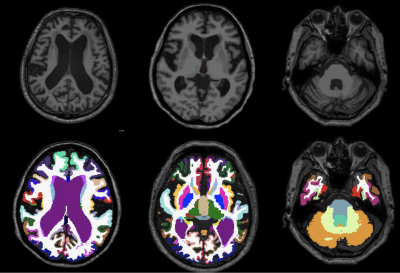
Figure 3. Axial T1W images (top row) and segmented brain using freesurfer (bottom row) used for ventricular volume evaluation in iNPH patient
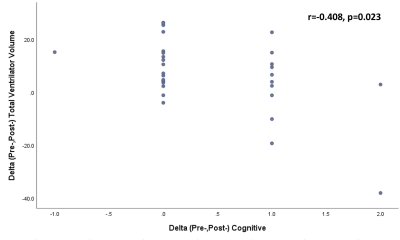
Figure 2. Correlation analysis between a reduction in ventricular volume and changes in iNPHGS scores in cognitive domain showing a moderate degree of a significant negative correlation (r = -0.408, p = 0.023).
-
Neuropathologic and Cognitive Correlates of Automatically Segmented Enlarged Perivascular Spaces in Community-based Older Adults
Carles Javierre-Petit1, Ashish A. Tamhane2, Arnold M. Evia2, Marinos Kontzialis2, Nazanin Makkinejad1, Gady Agam1, David A. Bennett2, Julie A. Schneider2, and Konstantinos Arfanakis1,2
1Illinois Institute of Technology, Chicago, IL, United States, 2Rush University Medical Center, Chicago, IL, United States
In this work, we first developed an algorithm to automatically
segment and quantify EPVS in brain MRI, and then investigated the
neuropathologic correlates of total and regional EPVS, as well as the
contributions of EPVS on cognitive decline in a large community-based cohort of
817 older adults.
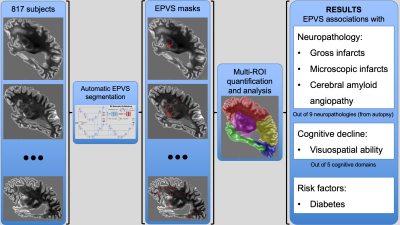
Figure 1. Study overview. From left to right, 817 ex-vivo participants
were preprocessed and their corresponding EPVS masks were generated
using a deep learning segmentation model. EPVS were quantified in the
whole brain and multiple ROIs to investigate the associations of EPVS
with neuropathologies, cognition, demographics and risk factors. This
study found EPVS to be associated with gross and microscopic infarcts,
cerebral amyloid angiopathy, cognitive decline in visuospatial ability,
and with diabetes.
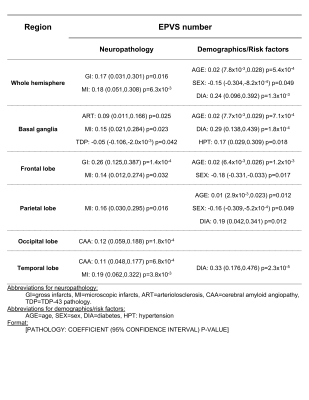
Figure 5. Neuropathologic, demographic, and risk factor associations of
quantified EPVS in each of the brain ROIs evaluated in this study.
Abbreviations and format shown at the bottom of the figure.
-
ALL CENTRAL NERVOUS SYSTEM (CNS) NEURO- AND VASCULAR-COMMUNICATION CHANNELS ARE SURROUNDED WITH CEREBROSPINAL FLUID (CSF)
Lara M Fahmy1, Yongsheng Chen2, Stephanie Xuan3, E Mark Haacke3, Jiani M Hu3, and Quan Jiang4
1Psychiatry and Behavioral Neurosciences, Wayne State University School of Medicine, Detroit, MI, United States, 2Neurology, Wayne State University School of Medicine, Detroit, MI, United States, 3Radiology, Wayne State University School of Medicine, Detroit, MI, United States, 4Neurology, Henry Ford Health System, Detroit, MI, United States
Our findings indicate that all peri-neural spaces surrounding all the cranial & spinal nerves & all peri-vascular spaces surrounding MRI-visible vasculature were filled with CSF; warranting further investigation into its cerebral waste clearance & immunomodulation implications.
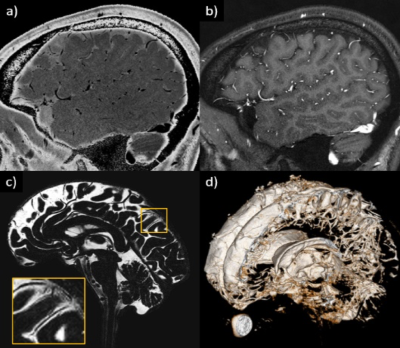
Figure 4. Illustration showing that all MRI-visible vasculature were surrounded by CSF. A) sT2W image with bright CSF and dark blood; B) T1W with bright blood signal but dark CSF signal; C) CSF-only images, acquired by T2-TSE sequence with TE = 345ms at 3T; D) a 3D rendering of the CSF-only images. A) and B) were results from the high-resolution STAGE scans. The enlarged image in C) shows several MRI-visible vasculature (dark curves) surrounded by bright CSF. Images were generated from the second experiment as described in the method section.
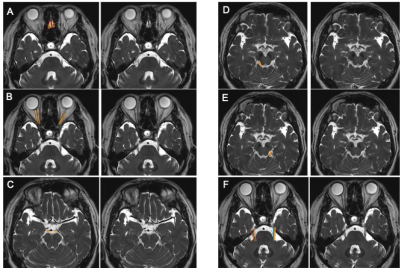
Figure 1. CSF-filled peri-neural spaces surrounding the first five cranial nerves (CN I-CN V). A) Olfactory nerves (CN I); B) Optic nerves (CN II); C) Oculomotor nerves (CN III); D) Right trochlear nerve (CN IV); E) Left CN IV; and F) Trigeminal nerves (CN V). Space within the yellow lines: respective nerve (hypointense signal). Space between the red and yellow lines: peri-neural space (hyperintense signal). Space between the blue lines: CN V (hypointense signal). All images were generated from the first experiment as described in the method section.
-
Association of Enlarged Perivascular Spaces and Sleep Disturbances in Military-related Mild Traumatic Brain Injury
Ping-Hong Yeh1, J. Kent Werner2,3, Rujirutana Srikanchana1, Kimbra Kenney1,2, Treven Pickett1,2, Grant Bonavia1,2, Gerard Riedy1,2, and John Ollinger1
1National Intrepid Center of Excellence, Bethesda, MD, United States, 2Uniformed Services University of the Health Sciences, Bethesda, MD, United States, 3Center for Neuroscience and Regenerative Medicine, Bethesda, MD, United States
Perivascular space (PVS) volume fraction in combat-related mild TBI (mTBI) patients with potential concussive events was associated with sleep measures with larger PVS fraction than controls. PVS dilatation may be modulated by sleep and traumatic brain injury.
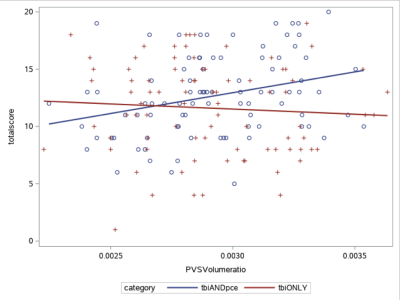
Fig. 4. Relationship between PVS volume fraction and PSQI score in mTBI. PVS volume fraction positively correlated with PSQI score in TBIPCE subgroup (tbiANDpce in blue, p=0.0026, r2= 0.13, blue), but not in TBIonly (tbionly in red) subgroup.
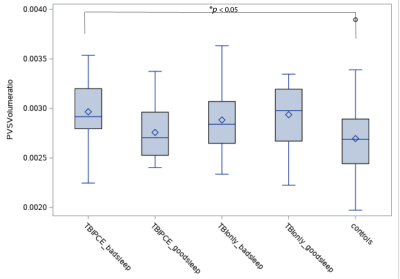
Fig. 3. Group comparisons of PVS volume fraction. TBIPCE subgroup had significantly higher PVS volume fraction than controls (F=6.0, 0.296% vs 0.273%, *corrected p=0.03, effect size: ω2 = 0.08 and η2 =0.1, calculated by setting the confidence coefficient equal to 10%, which is appropriate if the alpha for the F test is .05))
-
Cerebrospinal fluid water fraction increases with age in normal aging
Thanh D Nguyen1, Liangdong Zhou1, Elizabeth Sweeney1, Xiuyuan Wang1, Susan A Gauthier1, Yi Wang1, Amy Kuceyeski1, and Yi Li1
1Weill Cornell Medicine, New York, NY, United States
We applied FAST-T2 multi-component T2
relaxometry to 20 healthy volunteers between 30 and 60 years old and found a significant
relationship between CSF water fraction and age in the frontal and temporal
cortex, which may be related to the dilation of perivascular spaces in normal
aging.

Figure 2. Plots of CSF water
fraction (CSFF) change with age in the brain cortex and white matter of healthy
volunteers (n=20). Statistical significance was found in the frontal (p=0.0011)
and temporal (p=0.0093) cortex after correction for multiple comparisons.
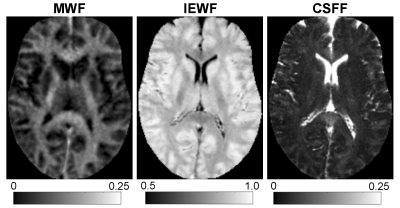
Figure 1. Example of quantitative maps of myelin water
fraction (MWF), intra/extracellular water fraction (IEWF), and CSF water
fraction (CSFF) obtained by FAST-T2 sequence in a 50-year-old female subject.
-
Diffusion and perfusion characteristics of brain white matter prone to hyperintensities: a four-year longitudinal study
Shruti Agarwal1, Jay J. Pillai1,2, and Hanzhang Lu3,4
1Division of Neuroradiology, Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Division of MR Research, Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4F. M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States
Areas of NAWM that develop WMH over time have reduced CBF at baseline
and these abnormalities progress faster than structural properties such as
diffusion or T1. CBF may provide an early marker for progression of age-related
WM disease.
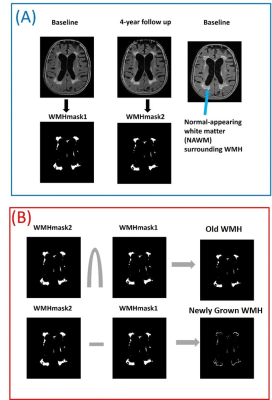
Figure
1: (A): White
matter hyperintense (WMH) regions are automatically segmented on FLAIR images
from baseline and follow-up and named as WMHmask1
and WMHmask2. Blue arrow shows the normal appearing white matter (NAWM) that
progressed to WMH in 4-year follow up. (B):
The intersection of WMHMask1 & WMHMask2 is referred as “Old WMH” at
baseline and the subtraction of WMHMask1 from WMHMask2 [(WMHMask2-WMHMask1)>0]
is referred as “Newly Grown WMH” at 4-year follow up (which was NAWM at
baseline).
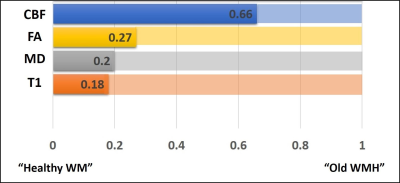
Figure 4: The relative values of diffusion,
perfusion, and T1 signal intensities among the three tissue types is displayed.
The bars are displayed such that the “Healthy WM” is on the left-end, the “Old
WMH” is displayed on the right-end, with the “Newly Grown WMH” displayed
proportionally along the bar. It can be seen that the characteristics of the
“Newly Grown WMH” are closer to the healthy tissue in all structural/anatomical
parameters, but their CBF resembles more to the “Old WMH” tissues.
-
Callosal Angle Biomarker for Normal Pressure Hydrocephalus Calculated for 4,980 T1-Weighted MR Exams
Alexander Saunders1,2, Stefan Bluml1,2, Kevin S King3, and Matthew Borzage2,4
1Radiology, Children's Hospital Los Angeles, Los Angeles, CA, United States, 2Rudi Schulte Research Insitute, Santa Barbara, CA, United States, 3Barrow Neurological Institute, Phenoix, AZ, United States, 4Children's Hospital Los Angeles, Los Angeles, CA, United States
Automatic callosal angle measurement can rapidly and objectively detect patients with normal pressure hydrocephalus. Automatic angle measurement calculated for 4,980 MRIs found that 1.8-4.3% of patients likely had normal pressure hydrocephalus.
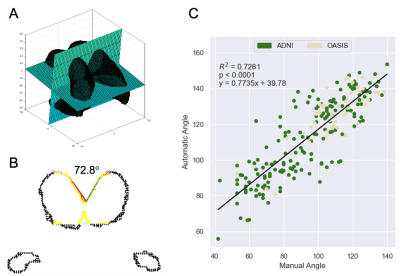
Figure 1 (A) Extracted ventricles with automatically placed oblique axial and coronal reference planes; (B) using the oblique slice from (A), the ventricle walls (black) are sliced, candidate points for the callosal wall are identified (yellow). Refiled points (red) indicate those included in the final calculation. Lines are then fit through the points on each side of the vertex and the angle is calculated; (C) reports the correlation between manual and automated measurements.
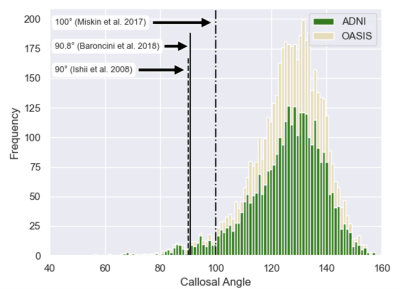
Figure 2 Histogram of 4,980 automatic callosal angle measurements. The data is skewed towards lower angles. Thresholds for suspected normal pressure hydrocephalus are indicated for several publications.
-
Perfusion and free water show opposite trends across tissue layers surrounding white matter hyperintensities in elderly participants
Corinne A. Donnay1, Pauline Maillard1, Charles DeCarli1, and Audrey P. Fan1,2
1Neurology, University of California Davis, Davis, CA, United States, 2Biomedical Engineering, University of California Davis, Davis, CA, United States
White matter hyperintensities and their surrounding tissue have lower perfusion and higher extracellular water, as reflected by free water, than healthy white matter. Cerebral blood flow increases from lower layers to higher layers. The opposite associations were found with free water.
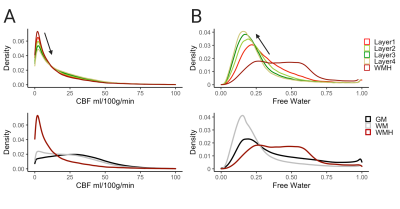
Figure 2. Summary density plots of all participants (n=19) and clusters (n=331) showing distribution differences between layers 1-4 and WMHs (top) and cortical tissue (bottom). (A) Distribution of CBF values (B) Distribution of FW values
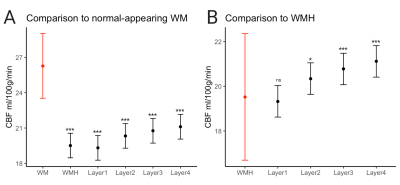
Figure 4. Results of linear mixed model comparing the mean CBF values of (A) WMHs and layers 1-4 to normal-appearing white matter, indicated in red and (B) layers 1-4 to WMHs, indicated in red.
***p<0.001, *p<0.01
-
Reduced cerebrovascular reactivity and cerebral blood flow in White Matter Hyperintensities (WMHs)
Chenyang Li1,2, Marco Muccio1, Dengrong Jiang3, Peiying Liu3, Jiangyang Zhang1, Arjun Masurkar4, Thomas Wisniewski4, Hanzhang Lu3, and Yulin Ge1
1Department of Radiology, Center for Biomedical Imaging, NYU Grossman School of Medicine, New York, NY, United States, 2Vilcek Institute of Graduate Biomedical Sciences, NYU Grossman School of Medicine, New York, NY, United States, 3Department of Radiology and Radiological Science, Johns Hopkins University, Baltimore, MD, United States, 4Department of Neurology, NYU Grossman School of Medicine, New York City, NY, United States
We implemented advanced neurovascular MRI techniques to evaluate the white matter hemodynamics including cerebral blood flow (CBF) and cerebrovascular reactivity (CVR) in patients with varying degrees of WMHs. Lower CBF and CVR function could be potentially associated with WMHs.
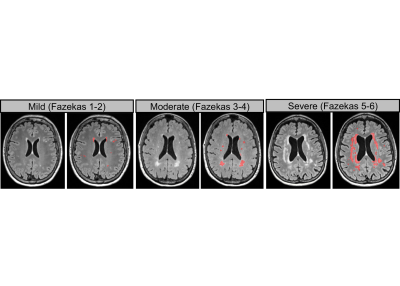
Figure 1.
Representation WMHs lesions on T2-FLAIR images in mild,
moderate and severe lesion groups and its corresponding lesion segmentation result (delineated in red).
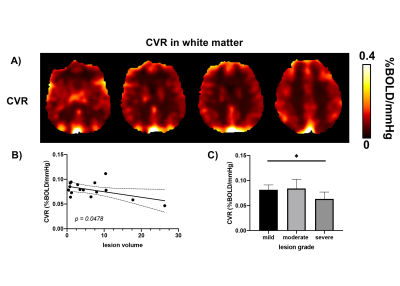
Figure 2.
A)
Representation of CVR maps in WMHs patients. B) Correlation analysis between lesion volume and white matter CVR. C) Group-wise comparison of white matter CVR between mild, moderate and severe lesion groups.
-
Decoupling between global brain activity and cerebrospinal fluid flow is associated with Alzheimer's disease pathologies
Feng Han1, Jing Chen1, Aaron Belkin-Rosen1, Yameng Gu1, Liying Luo2,3, Orfeu M Buxton4, and Xiao Liu1,5
1Department of Biomedical Engineering, The Pennsylvania State University, State College, PA, United States, 2Department of Sociology & Criminology, The Pennsylvania State University, State College, PA, United States, 3Population Research Institute, The Pennsylvania State University, State College, PA, United States, 4Department of Biobehavioral Health, The Pennsylvania State University, State College, PA, United States, 5Institute for Computational and Data Sciences, The Pennsylvania State University, State College, PA, United States
The study used the coupling between the global BOLD signal and CSF flow as a surrogate marker for gauging glymphatic function, and found significant correlations between this coupling metric and cognitive and molecular markers of AD.
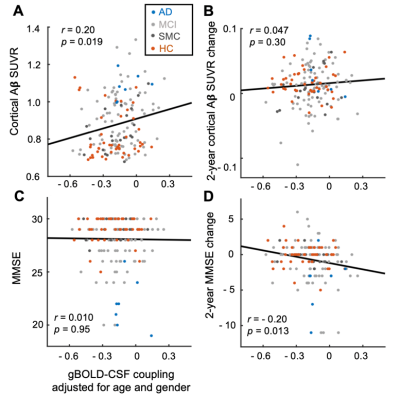
Fig. 3. The gBOLD-CSF coupling is correlated with cortical Aβ and cognitive decline. The age-&gender-adjusted gBOLD-CSF coupling strength decreased with increasing cortical Aβ SUVRs at baseline (A) but not their changes in the following two years (B). This gBOLD-CSF coupling is significantly correlated with the 2-year MMSE score changes (C) but not with its baseline (D). The linear regression lines were estimated based on the linear least-squares fitting. Each dot represents a single session.
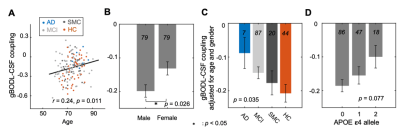
Fig. 2. The dependency of the gBOLD-CSF coupling on AD risk factors and disease conditions. The strength of the gBOLD-CSF coupling (global BOLD-CSF correlation at +3 sec lag) decreased with age (A; Spearman’s r = 0.24, the linear mixed model with Satterthwaite's method17) and in female participants (B). This gBOLD-CSF coupling (age-&gender-adjusted) also decreased with AD development (C) and with the APOE 𝛆4 allele number carrying (D). Error bar: SEM; session number showed in each bar.
-
Cerebrovascular Reactivity and Cerebral Blood Flow across lifespan in females
Safa Sanami1, Brittany Intzandt2,3,4, Fatemeh Razavipour1, Julia Huck1, Richard D Hoge5, Louis Bherer3,4,6,7, and Claudine J Gauthier1,4,6
1Physics, Concordia University, Montreal, QC, Canada, 2INDI, Concordia University, Montreal, QC, Canada, 3Centre de Recherche de l'Institut Universitaire de Geriatrie, Montreal, QC, Canada, 4Centre de Recherche, l'Institut de Cardiologie de Montréal, Montreal, QC, Canada, 5Department of Neurology and Neurosurgery, McGill University, Montreal, QC, Canada, 6PERFORM Centre, Concordia University, Montreal, QC, Canada, 7Départment de Médicine, Université de Montréal, Montreal, QC, Canada
Cerebral blood flow and cerebrovascular
reactivity decline in females every decade during healthy aging.
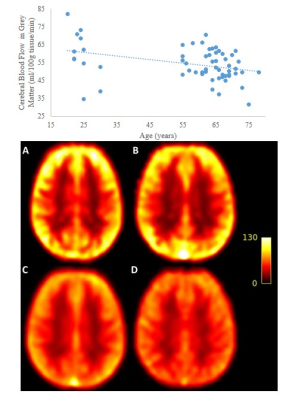
Figure 1: Mean CBF
(ml/100g/min) for females across decades:
A- 20 to 29 yo; B- 50 to 59
yo; C- 60 to 69 yo; D – 70 to 79 yo. The graph demonstrates the relationship
between age and CBF.
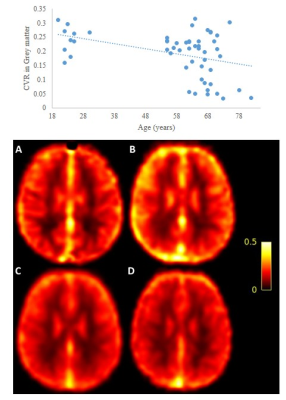
Figure 2: Mean CVR (ml/100g/min/ΔmmHg CO2) for females across
decades:
A - 20 to 29 yo; B- 50 to
59 yo; C- 60 to 69 yo; D – 70 to 79 yo. The graph demonstrates the relationship
between age and CVR.
-
Changes of Choroid Plexus Volume in Alzheimer’s Patients
Li Zhao1, Xue Feng2, Yueqin Hu3, Dan Wu1, Craig H. Meyer2, and David C. Alsop4
1College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, China, 2Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 3Psychology, Beijing Normal University, Beijing, China, 4Radiology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, United States
This is a study of the choroid plexus volume using a deep learning method. We found that the choroid plexus volume 1) increases with age; 2) is smaller in female subjects; and 3) is larger in MCI and AD patients compared to healthy volunteers.
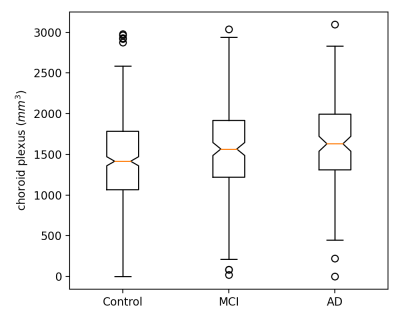
Figure 4 Significantly smaller choroid plexus volume in healthy volunteers compared to the MCI and AD patients
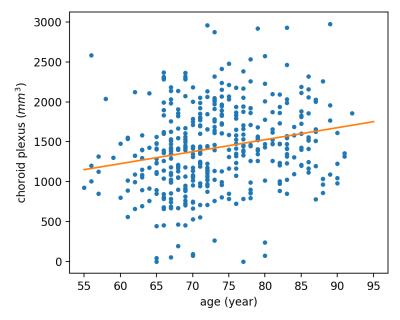
Figure 2 Choroid plexus volume changes with age in healthy volunteers
-
Effect of white matter hyperintensities on tractometry and its relationship with white matter connectivity
Tae Kim1, Howard J Aizenstein1, and James T Becker1
1University of Pittsburgh, Pittsburgh, PA, United States
Elevated WMHs load on white matter tract was associated with lower apparent fiber density (AFD) on tractometery, while reduced AFD is associated with lower connectivity of the WM tract.
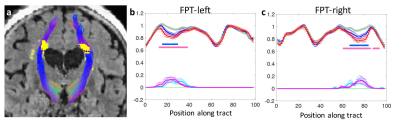
Fig.1. The obtained fronto-pontine tract are overlaid on a FLAIR image with corresponding group-averaged left (b) and right (c) tract-profiles. (a) Yellow binary masks: WMH segmentations. The tracts are passing through WMH. (b,c) The AFD tract profiles (upper) of higher WMH (blue) and MCI (red) groups are statistically significant compared to those of lower WMH group (dark green) at the location of WMH in WMH tract profiles (lower, light green: lower WMH, light blue: higher WMH, magenta: MCI groups). Error bars: S.E.M. Asterisk marks: p < 0.05 (blue: higher WMH, pink: MCI groups).

Fig. 3. The comparisons of the amount of WMH (a), mean AFD (b), and connectivity (c) on tractometry between lower WMH, higher WMH and MCI groups. Differences between groups was statistically significant for each comparison across all groups. (p < 0.0001). The central marks of box plots indicate the median, and the bottom and top edges of the box indicate the 25th and 75th percentiles, respectively. The whiskers extend to the most extreme data points not considered outliers, and the outliers are plotted individually using the '+' symbol
-
Cerebro spinal fluid dynamic in front of cardiac and breathing influence
Olivier Baledent1, Pan Liu1, Serge Metanbou1, Cyrille Capel1, Sidy Fall1, and Roger Bouzerar1
1university hospital Jules Verne, Amiens, France
Echo planar Phase Contrast can quantify CSF flows in acceptable conditions without cardiac or respiratory gating.
Even if physiological
breathing modulate only the CSF flows signals by around 10%, the cardiac power
is the main force able to change the direction of the flow during cardiac cycle.
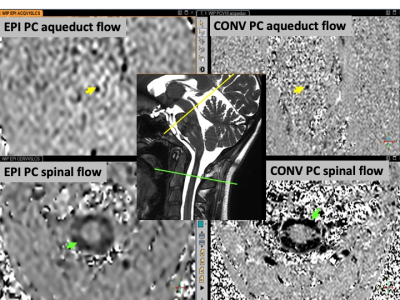
CSF flows in the aqueduct and spinal spaces.
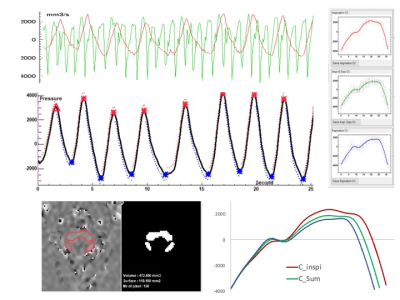
CSF flows continously acquired by EPI PC at the spinal level.
Base on the respiratory signal, EPI PC signal can be post processed. CSF flow dynamic is reconstructed along cardiac cyle during inspiration (red) and expiration (blue) periods. CSF flows oscillate around the zero line during each crdiac cycle.
-
UPSS - Unsupervised perivascular spaces segmentation method with salient guidance of frangi filter
Haoyu Lan1 and Farshid Sepehrband1
1USC Stevens Neuroimaging and Informatics Institute, University of Southern California, Los Angeles, CA, United States
In this work, we proposed an unsupervised learning method for perivascular spaces segmentation by combing filter-based image processing technique and deep learning algorithm. The hybrid method improved the segmentation performance and eliminated the needed for manual annotation
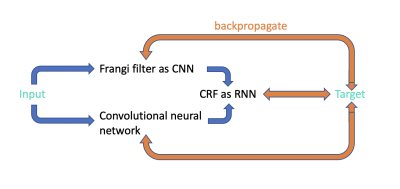
Figure 1. The architecture of the proposed unsupervised learning segmentation method UPSS. UPSS is composed of mainly two parts: a Frangi filter as convolution neural network (CNN) with fixed gaussian kernels and a simple convolutional neural network like Unet. The results from these two parts are used as inputs of a conditional random filed (CRF) as the recurrent neural network (RNN) to perform segmentation post-processing. The three parallel backpropagations are conducted during each training step to effectively train all the weights and parameters of UPSS.
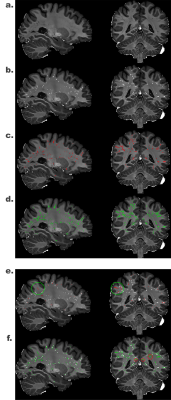
Figure2. Qualitative assessment of segmentation results of Unet and UPSS. The input image a., Frangi filter result b., UPSS result c., Unet result d., comparison between Frangi filter result and UPSS result e., comparison between Frangi filter result and Unet result f.
