-
DeepCEST: 7T Chemical exchange saturation transfer MRI contrast inferred from 3T data via deep learning with uncertainty quantification
Leonie E. Hunger1, Alexander German1, Felix Glang2, Katrin M. Khakzar1, Nam Dang1, Angelika Mennecke1, Andreas Maier3, Frederik Laun4, and Moritz Zaiss1,2
1Department of Neuroradiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 2High-field Magnetic Resonance Center, Max Planck Institute for Biological Cybernetics, Tübingen, Germany, 3Pattern Recognition Lab, Friedrich-Alexander-University Erlangen-Nürnberg, Erlangen, Germany, 4Institute of Radiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany
The deepCEST approach enables to perform a CEST experiment at 3T and
predict the contrasts of a CEST experiment at 7T with a neural network, including an uncertainty
quantification.
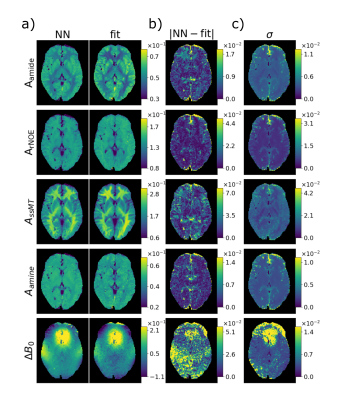
Figure 2: DeepCEST network trained on
healthy volunteers applied on a healty volunteer (a) Comparison of the
predictions and the Lorentzian fit amplitudes (ground truth). (b) Error of the
prediction compared to the fit. (c) Uncertainty of the prediction.
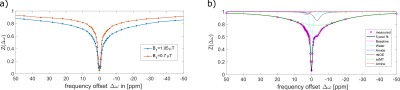
Figure 1: a) Input data: uncorrected Z-spectra of two B1 levels acquired at 3T. b) Target data: 5-pool-Lorentzian fitted data
acquired at 7T. As targets only the Lorentzian amplitudes were used.
-
Reduction of 7T CEST scan time and evaluation by L1-regularised linear projections
Moritz Simon Fabian1, Felix Glang2, Katrin Michaela Khakzar1, Angelika Barbara Mennecke1, Alexander German1, Manuel Schmidt3, Burkhard Kasper4, Arnd Dörfler1, Frederik B. Laun1, and Moritz Zaiss1
1Department of Neuroradiology, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nürnberg, Erlangen, Germany, 2High-field Magnetic Resonance Center, Max Planck Institute for Biological Cybernetics, Tübungen, Germany, 3Department of Neuroradiology, University Hospital Erlangen, Erlangen, Germany, 4Department of Neurology, Epilepsy Center, University Hospital Erlangen, Erlangen, Germany
The L1-regularized linear
projection approach allows for reduction of scan time by a factor of ~2.8,
while still being able to predict multiple CEST contrast parameters. It
generalizes to unseen healthy subject data and tumor patient data.
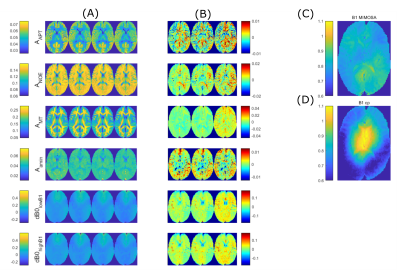
Figure
2: Comparison of retrospective and prospective input feature reduction as well
as conventional Lorentzian fitting. (A)
left to right: ground truth by Lorentzian fitting on full measurement, linear
prediction with all input features contained, linear prediction with retrospective
reduction of input features according to the LASSO-reduced sampling schedule,
measurement with these input features being prospectively omitted. (B) Difference maps of the linear
predictions and the ground truth. (C)
B1-MIMOSA and (D) B1-cp map as mentioned
in 5
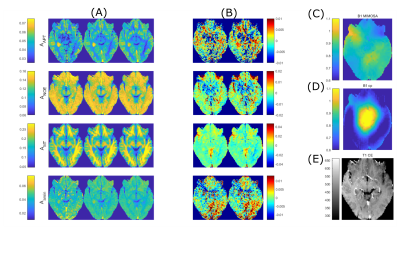
Figure 3: Comparison of
retrospective reduction of input features for a tumor patient dataset
(Glioblastoma WHO grade 4). (A) left
to right: ground truth obtained by Lorentzian fitting, linear prediction with
all input features retained, retrospective reduction of input features. (B) Difference maps between linear
predictions and ground truth. (C) B1
MIMOSA map and (D) B1-cp map as mentioned
in 5. (E) Anatomical T1
contrast-enhanced image
-
Open source Pulseq interpreter for CEST MRI on Bruker systems
Sebastian Mueller1, Kai Herz1, Klaus Scheffler1,2, and Moritz Zaiss1,3
1High-field Magnetic Resonance Center, Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 2Department of Biomedical Magnetic Resonance, Eberhard Karls University Tuebingen, Tuebingen, Germany, 3Department of Neuroradiology, University Hospital Erlangen, Erlangen, Germany
The proposed approach allows straightforward
implementation of CEST MRI on Bruker scanners without the need of sequence
programming in C. Pre-saturation is defined in open source pulseq-files which
allows direct transfer to clinical devices or usage for simulations.
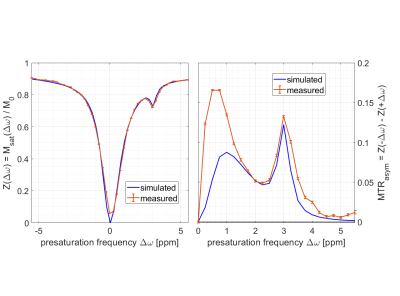
Figure 2:
Data measured (spatial mean and SD) at
B0 = 14T and simulations (same pulseq-file). CEST pools included
water (T1=1735ms, T2=18ms, f=100%), ssMT (T1=1000ms,
T2=0.01ms, dω=0ppm, kex=23Hz, f=5%), L-arginine (T1=1000ms,
T2=5ms, dω=3ppm, f=2.25%) and agar-agar (10) (T1=1000ms, T2=50ms,
dω=1.6ppm, kex=6500Hz, f=1%). Details on pre-saturation and simulation source code: https://pulseq-cest.github.io/
file “APTw_3T_002”; modifications: dω list and B0. Larger deviations in
MTR assymetry at <= 1.5 ppm are most likely due to residual B0
variation in the measured data.
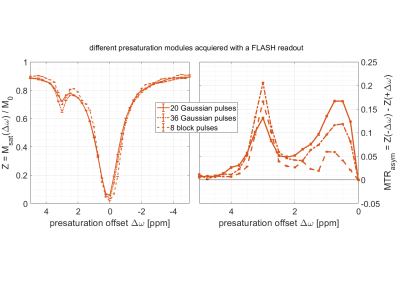
Figure 4: (left)
ROI averaged (mean ± SD) Z-spectra
acquired for different CEST pres-aturation modules using Bruker’s FLASH readout. (right) MTR asymmetry.
-
Linear projection-based CEST reconstruction – the simplest explainable AI
Felix Glang1, Moritz Fabian2, Alex German2, Katrin Khakzar2, Angelika Mennecke2, Frederik Laun3, Burkhard Kasper4, Manuel Schmidt2, Arnd Doerfler2, Klaus Scheffler1,5, and Moritz Zaiss1,2
1High-field Magnetic Resonance Center, Max Planck Institute for Biological Cybernetics, Tübingen, Germany, 2Department of Neuroradiology, University Hospital Erlangen, Erlangen, Germany, 3Institute of Radiology, University Hospital Erlangen, Erlangen, Germany, 4Neurology, Epilepsy Center, University Clinic of Friedrich Alexander University Erlangen-Nürnberg, Erlangen, Germany, 5Department of Biomedical Magnetic Resonance, Eberhard Karls University Tübingen, Tübingen, Germany
Linear projection-based CEST
reconstruction allows mapping from acquired raw in vivo CEST data to multiple
contrast parameters of interest, generalizing from healthy subject training
data to unseen test data of both healthy subjects and tumor patients.
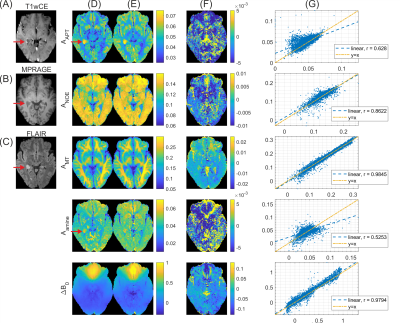
Figure
4. Results of linear projection in a tumor patient test
dataset. Clinical contrasts: (A) T1 weighted contrast-enhanced, (B) MPRAGE and
(C) FLAIR. (D) Ground truth Lorentzian fit results. (E) Contrast maps obtained
by linear projection with coefficients obtained from 5 healthy subject
datasets. (F) Difference maps to ground truth for linear projection result. (G)
Voxel-wise scatter plots of linear prediction result (y) versus ground truth (x) with
legends indicating Pearson correlation coefficient r.
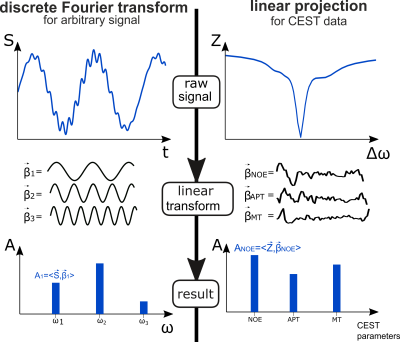
Figure
1. Analogy of discrete Fourier transform and the proposed
linear projection approach for CEST evaluation. For the Fourier transform, a
signal vector S(t) is projected onto basis vectors $$$\beta_1,...,\beta_n$$$ consisting of the respective harmonics to
yield the Fourier coefficients $$$A_1,...,A_n$$$ for different frequencies. For linear CEST
evaluation, acquired raw data are projected onto coefficient vectors found by linear regression applied to conventionally evaluated data, to yield target contrasts like APT, NOE and ssMT amplitudes.
-
In-Vivo Sub-Minute rNOE Mapping Using AutoCEST: a Machine-Learning Approach for CEST/MT Protocol Invention and Quantitative Reconstruction
Or Perlman1, Bo Zhu1,2, Moritz Zaiss3,4, Naoyuki Shono5, Hiroshi Nakashima5, E. Antonio Chiocca5, Matthew S. Rosen1,2, and Christian T. Farrar1
1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 2Department of Physics, Harvard University, Cambridge, MA, United States, 3Magnetic Resonance Center, Max Planck Institute for Biological Cybernetics, Tübingen, Germany, 4Department of Neuroradiology, University Clinic Erlangen, Erlangen, Germany, 5Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, United States
A machine-learning framework was
expanded
for simultaneously designing the optimal CEST protocol
and extracting fully quantitative maps in-vivo. A mouse tumor
rNOE volume-fraction was significantly decreased, in agreement with
previous human studies.
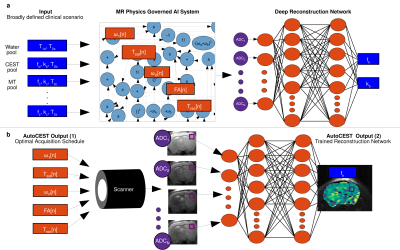
Fig. 1 AutoCEST pipeline. a.
Pre-experiment step. The broad expected range of properties (blue
rectangles) are given as input to an MR physics governed AI system,
which dynamically optimizes the protocol parameters (orange
rectangles). The resulting “ADC” MR signals are then decoded into
quantitative parameters using a deep
reconstruction network. b.
Experiment step. The optimal schedule parameters are loaded into the
scanner, resulting in a set of N raw images. The resulting images are
fed voxelwise into the trained reconstruction network, resulting in
quantitative CEST maps.
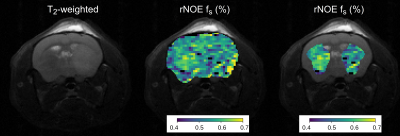
Fig 5 In vivo study results. (Left).
T2-weighted image of a tumor-bearing mouse. (Center). The
corresponding rNOE volume fraction quantitative map (fs),
and its overlay (right) atop the tumor and contralateral ROIs. Note
the decreased fs
at the tumor.
-
Whole-Brain Steady-State CEST at 3T Using MR Multitasking
Pei Han1,2, Karandeep Cheema1,2, Hsu-Lei Lee1, Zhengwei Zhou1, Tianle Cao1,2, Sen Ma1, Nan Wang1, Anthony G. Christodoulou1,2, and Debiao Li1,2
1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Department of Bioengineering, UCLA, Los Angeles, CA, United States
We propose a fast 3D steady-state CEST method at
3T using MR Multitasking. By exploiting the correlation among images
throughout the spatial, time, and offset frequency dimensions with a low-rank
tensor, the Z-spectrum acquisition with whole-brain
coverage can be done within 5.5min.
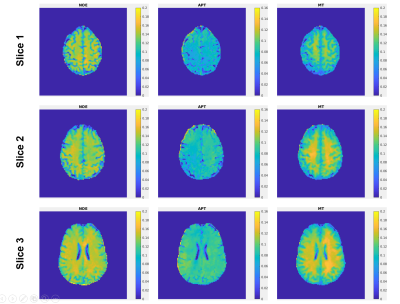
Figure 2: Representative NOE, APT
and MT maps from Multitasking ss-CEST. Three different slices are displayed to
show the consistency of image quality within the 3D volume.
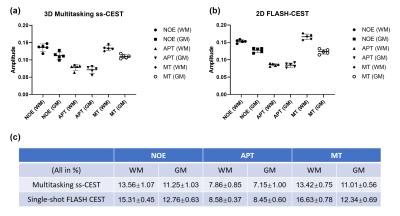
Figure 4: Average Lorentzian
amplitudes within GM and WM regions among different volunteers. The mean
amplitude is consistent among healthy subjects. Contrast ratios of WM:GM for
NOE/APT/MT: 1.21/1.10/1.22 (Multitasking ss-CEST) vs. 1.20/1.02/1.35 (2D
single-shot FLASH CEST).
-
Deep Learning-based image reconstruction improves CEST MRI
Shu Zhang1, Xinzeng Wang2, F. William Schuler1, R. Marc Lebel3, Mitsuharu Miyoshi4, Ersin Bayram2, Elena Vinogradov5, Jason Michael Johnson6, Jingfei Ma7, and Mark David Pagel1,7
1Cancer Systems Imaging, MD Anderson Cancer Center, Houston, TX, United States, 2Global MR Applications & Workflow, GE Healthcare, Houston, TX, United States, 3Global MR Applications & Workflow, GE Healthcare, Calgary, AB, Canada, 4Global MR Applications & Workflow, GE Healthcare Japan, Tokyo, Japan, 5Radiology, UT Southwestern Medical Center, Dallas, TX, United States, 6Neuroradiology, MD Anderson Cancer Center, Houston, TX, United States, 7Imaging Physics, MD Anderson Cancer Center, Houston, TX, United States
Deep
learning-based image reconstruction (DL Recon) substantially reduced the noise
in the CEST maps and improved the lesion conspicuity.
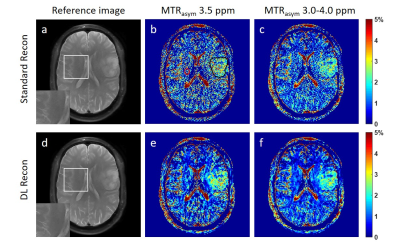
Figure 1. The reference images using standard
recon (a) and DL Recon (d). The MTRasym map at 3.5 ppm (b,e) and
averaged between 3.0-4.0 ppm (c,f) using standard recon (b,c) and DL recon
(e,f) of a glioma patient. The tumor ROI (black) and the background ROI of
homogenous brain tissue (red) was shown in (a) as an example.
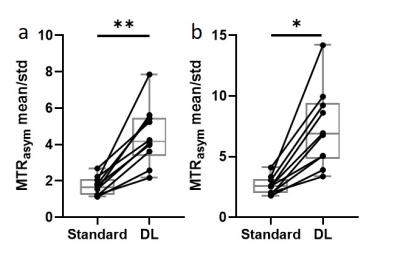
Figure 3. The tumor ROI-averaged MTRasym divided by the standard
deviation of the background ROI at 3.5 ppm (a) and 3.0-4.0 ppm (b) using
standard recon and DL Recon were compared for all patients. (a) p = 0.002. (b)
p = 0.016. **: p < 0.01. *: p < 0.05.
-
Indirect Inference of Acidification in Exercised Skeletal Muscle using Creatine CEST
Dushyant Kumar1, Ryan Armbruster1, Neil Wilson2, Ravi Prakash Reddy Nanga1, and Ravinder Reddy1
1Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Siemens Medical Solutions USA Inc, Malvern, PA, United States
We demonstrate the feasibility of indirect
detection of acidification in exercised skeletal muscle using creatine CEST
findings from healthy volunteers.
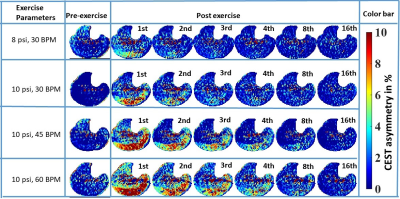
Fig. 1: ΔCrCEST
time-series for volunteer #1, corresponding to the middle slice (5th
out of 8 slices). Each row corresponds to different exercise workload. Selected time frames of post-exercise CrCEST
time-series are being presented, with frame number written on right top corner
of each frame. The temporal resolution was 30s. Though
at mild PFE level, the volunteer mostly utilized the lateral gastrocnemius
(LG), both LG and medial gastrocnemius (MG) were utilized during intense
PFE. As evident visually, the recovery
rate increased with increased intensity of exercise levels.
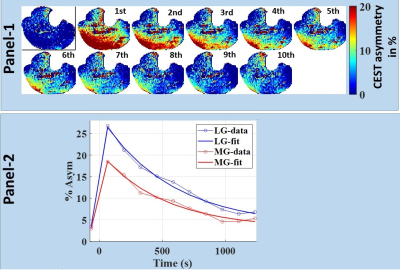
Fig. 2:
Panel -1: Time-series of Lactate CEST (LATEST) maps show intense
exercise-induced changes in skeletal muscle, using images from 5th slice of volunteer #1. First LATEST frame corresponds to
pre-exercise level, whereas the remaining ten frames correspond to ten time points
post-exercise with 140s temporal resolution. Panel-2:
Time-series plots of LATEST values averaged over the entire lateral
gastrocnemius and corresponding fits are shown.
-
Simultaneous mapping of B0, B1 and T1 for the correction of CEST-MRI
Kerstin Heinecke1, Henrik Narvaez1, Christoph Kolbitsch1, and Patrick Schuenke1
1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany
We developed a new method for simultaneous B0-,
B1- and T1- mapping, using a simple adapted CEST sequence.
First results prove the feasibility of this approach and its applicability for
the correction of CEST-MRI data.
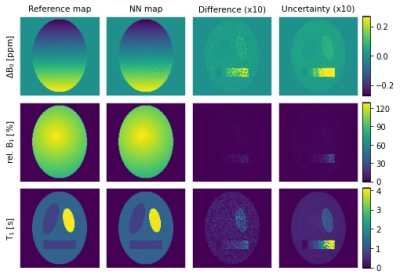
Parameter maps and uncertainty estimation of
simulated phantom data. The left column shows the reference maps with
simulated B0- and B1- inhomogeneities and different
brain-matter-like T1 values with 1% Gaussian noise overall and one compartment
with noise ranging from 0% to 10%. From second left to right: maps generated by
the NN, difference maps between reference and NN output (x10) and uncertainty
maps generated by the NN (x10).
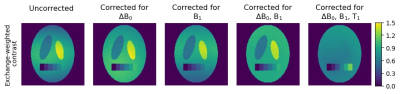
Exchange-weighted contrasts of simulated CEST
data before and after post-processing. Simulated with B0- and B1-
inhomogeneities, different brain-matter-like compartments and a
three-pool-system with the same CEST-pool fraction of f = 0.6% overall and one compartment
with fractions ranging from 0% to 1%. Left to right: MTRRex7
contrasts of i) the uncorrected CEST-simulation and after ii) ΔB0-correction5, iii) B1-correction6,
iv) ΔB0- and B1-correction.
Right: AREX contrast (ΔB0-, B1- and T1-corrected)7.
-
Quasi-steady-state (QUASS) CEST solution improves the accuracy of CEST quantification – QUASS CEST MRI-based omega plot analysis
Phillip Zhe Sun1
1Department of Radiology and Imaging Sciences, Emory University, Atlanta, GA, United States
The QUASS CEST analysis accounts
for the effect of finite Ts and Td and improves the accuracy of CEST MRI
quantification. Both simulation and CEST MRI experiments confirmed that the
QUASS solution enabled robust quantification of ksw and
fr, superior over the conventional apparent CEST MRI.
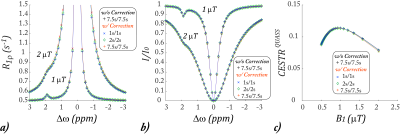
Fig. 2., Simulation of the proposed QUASS CEST MRI. a) R1rho determined from
the QUASS solution for three representative sets of Td and Ts (i.e., 1s/1s
(cross markers), 2s/2s (diamond markers), and 7.5s/7.5s (plus markers) and that
calculated under long Td and Ts (7.5s/7.5s, black plus markers) for B1
levels of 1 and 2 µT. b) The QUASS Z-spectra
under the three representative sets of Td and Ts and two B1 levels
of 1 and 2 µT. c) The QUASS CEST effect at 1.9 ppm as a function of B1
level for three sets of Ts and Td times, being 1s/1s, 2s/2s, and 7.5s/7.5s.
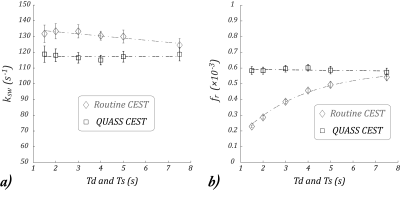
Fig. 4., The relationship between Td/Ts with the
experimentally determined ksw
and fr. a) ksw from the conventional CEST MRI (circle markers) showed a significant linear dependence with Ts/Td. In
comparison, ksw determined
from the QUASS CEST MRI (square markers) did not show significant dependence with respect to Ts/Td.
c) fr determined from conventional
apparent CEST MRI (circle markers) can be described by a stretched exponential function with
significant dependence on Ts/Td. d) fr
determined from the QUASS CEST MRI (square markers) showed no significant dependence on Ts/Td.
-
MTC removed and exchange rate differentiated CEST using Variable Delay Multi Pulse (VDMP) in the human brain at 7T
Bárbara Schmitz Abecassis1, Elena Vinogradov Vinogradov2,3, Jannie P. Wijnen4, Thijs van Harten1, Evita C. Wiegers4, Hans J.M. Hoogduin4, Matthias J.P. van Osch 1, and Ece Ercan1
1Department of Radiology, Leiden University Medical Center, Leiden, Netherlands, 2Department of Radiology, UT Southwestern Medical Center, Dallas, TX, United States, 3Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 4Department of Radiology, University Medical Center Utrecht, Utrecht, Netherlands
Variable delay multi pulse (VDMP) CEST imaging at 7T allowed identifying slow and fast exchanging solute pools present in the human brain in vivo, by means of saturation build-up curves upon MTC removal and evaluation of optimal B1 amplitudes.
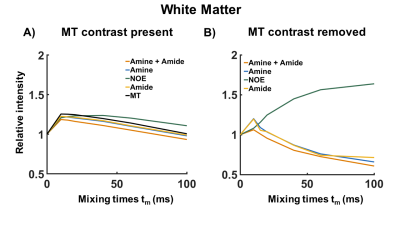
Figure 4. A typical example of the effect of MTC on the VDMP build-up curves from the human WM for B1 = 1.99µT. In A) the saturation build-up of the different CEST-pools is plotted together with the MT contrast. In B) the MTC has been removed and the trend of the VDMP build-up curves from the CEST-pools with different exchange rates can be distinguished.
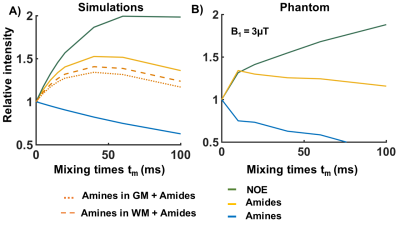
Figure 2. Normalized VDMP saturation build-up curves for different mixing times. A) Simulated build-up curves for CEST-pools found in the human brain: according to the concentration of amines in the GM (dotted line) and WM (dashed line) B) Build-up curves from glutamate (amines in blue) and bovine serum albumin phantoms (amides in yellow and rNOE in green). Both simulation and phantom data show a gradual build-up for slow exchanging molecules (rNOE and amides) as opposed to a fast decay from fast exchanging amines.
-
Highly Accelerated 1mm3-Isotropic 3D CEST MRI with Spectral Random Walk CAIPIRINHA Sampling at 7T
Sugil Kim1, Seong-Gi Kim2,3, and Suhyung Park4,5
1Siemens Healthineers, Seoul, Korea, Republic of, 2Center for Neuroscience Imaging Research (CNIR), Institute for Basic Science (IBS), Suwon, Korea, Republic of, 3Department of Biomedical Engineering, Sungkyunkwan University, Suwon, Korea, Republic of, 4Department of Computer Engineering, Chonnam National University, Gwangju, Korea, Republic of, 5Department of ICT Convergence System Engineering, Chonnam National University, Gwangju, Korea, Republic of
We propose highly accelerated 3D CEST MRI using
CAIPI sampling based 3D segmented EPI with spectral random walk, potentially enabling
1mm-isotropic whole-brain CEST imaging within 5min at 7T.
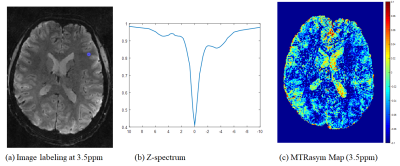
Fig3
(a) CEST labeling image at 3.5ppm, (b) Z-spectrum with marked as blue circle in
(a), and (c) MTR asymmetric map at 3.5ppm.
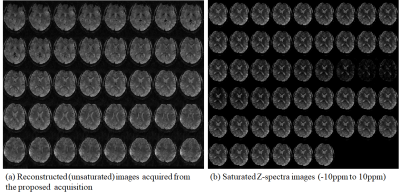
Fig2
(a) Reconstructed (unsaturated) images acquired from the proposed acquisition, (b) Saturated Z-spectra images offset frequencies -10ppm to 10ppm
-
Mapping of intracellular pH in vivo using amide and guanidyl CEST-MRI at 9.4 T
Philip S Boyd1, Johannes Breitling1, Stephanie Laier2, Karin Mueller-Decker2, Andrey Glinka3, Mark E Ladd1, Peter Bachert1, and Steffen Goerke1
1Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Center for Preclinial Research, Core Facility Tumor Models, German Cancer Research Center (DKFZ), Heidelberg, Germany, 3Division of Molecular Embryology, German Cancer Research Center (DKFZ), Heidelberg, Germany
The
presented method allows calculation of reliable pH maps in the presence of
varying concentration, superimposing CEST signals, magnetization transfer and
spillover dilution. Applicability in vivo was demonstrated in mice showing an
average intracellular pH in tumors of approximately 7.
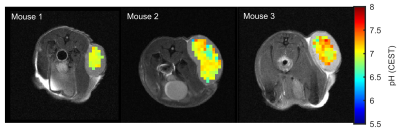
Figure 3:
In vivo application of the calibrated pH-CEST technique in tumor-bearing mice. pH
values of 6.97 ± 0.09, 7.00 ± 0.20 and 7.13 ± 0.19 were found in the subcutaneous lesions
of three DLD xenografted nude mice.
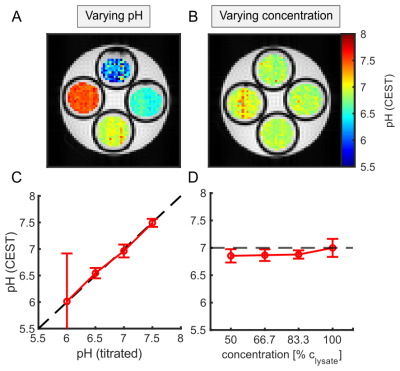
Figure 2:
Correlation of calculated pH from CEST-MRI with titrated pH (A,C) and tissue
concentration (B,D) of porcine brain lysate. C: Correlation of the titrated pH
and the mean pH values calculated from the CESTratio. D: Mean pH
values calculated from the CESTratio as a function of concentration.
-
7 tricks for 7T CEST: improving the reproducibility of multi-pool evaluation
Angelika Mennecke1, Katrin Khakzar1, Kai Herz2, Moritz Fabian1, Alexander German1, Andrzej Liebert3, Ingmar Blümcke4, Burkhard Kasper5, Manuel Schmidt1, Arnd Dörfler1, Armin Nagel3, Frederik Laun3, and Moritz Zaiß1
1Department of Neuroradiology, University Hospital of Erlangen, FAU Erlangen-Nürnberg, Erlangen, Germany, 2High-field Magnetic Resonance Center, Max Planck Institute for Biological Cybernetics, Tübingen, Germany, 3Institute of Radiology, University Hospital of Erlangen, FAU Erlangen-Nürnberg, Erlangen, Germany, 4Institute of Neuropathology, University Hospital of Erlangen, FAU Erlangen-Nürnberg, Erlangen, Germany, 5Department of Neurology, Epilepsy Centre, University Hospital of Erlangen, FAU Erlangen-Nürnberg, Erlangen, Germany
With the help of the presented post-processing procedure, it is possible to
obtain an increased reproducibility. For the amide contrast, the CoV decreased from 4% to less than
1.2 %.
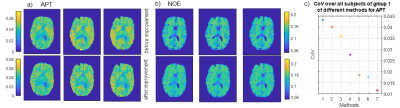
a) APT maps and b) NOE maps of a repeated measurement (group 1) before and after improving the post-processing steps (colorbar adjusted for visual comparison) c) decreasing CoV as a function of the stepwise improvement of post-processing methods: 1) moco with interpolation, 2) additionally, 2 point M0 normalization, 3) moco to the offset at 3.5 ppm, 4) B0 correction by using a Lorentzian fit 5) B0 correction with spline interpolation parameter 0.999 6) 2 point MZ normalization 7) LFM 4
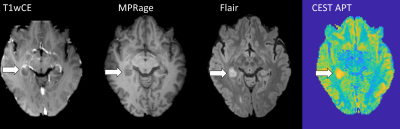
Conventional
images (contrast-enhanced T1 weighted imaging, MPRage, and Flair) and CEST APT
image of an epilepsy-associated tumor initially diagnosed as low grade,
histologically proven to be glioblastoma, IDH wild-type. Improved CEST postprocessing pipeline
was used.
-
Pulseq-CEST: Towards multi-site multi-vendor compatibility and reproducibility of CEST experiments using an open source sequence standard
Kai Herz1,2, Sebastian Mueller1, Maxim Zaitsev3, Linda Knutsson4,5, Jinyuan Zhou5, Phillip Zhe Sun6, Peter van Zijl5,7, Klaus Scheffler1,2, and Moritz Zaiss1,8
1Magnetic Resonance Center, MPI for Biological Cybernetics, Tuebingen, Germany, 2Biomedical Magnetic Resonance, University of Tuebingen, Tuebingen, Germany, 3Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Vienna, Austria, 4Medical Radiation Physics, Lund University, Lund, Sweden, 5Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 6Yerkes Imaging Center, Emory University, Atlanta, GA, United States, 7F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 8Neuroradiology, Friedrich‐Alexander Universität Erlangen‐Nuernberg, Erlangen, Germany
Pulseq-CEST enables a straightforward
approach to standardize, share, simulate and measure different CEST preparation
schemes, which are inherently completely defined.
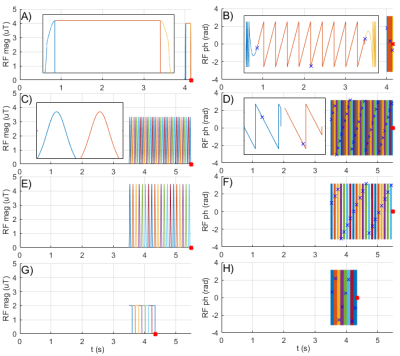
RF magnitude A) and phase B) of
the spin-lock saturation at 0.6 ppm. The frequency modulation can
be seen from the changing phase in the zoomed plot (black rectangle). The red dot
marks the beginning of the readout period. RF magnitude (C,E,G) and phase
(D,F,H) during three different APTw protocols: APTw_3T_001 (C,D), APTw_3T_002
(E,F) and APTw_3T_003 (G, H) all with B1,cwpe = 2 µT and recovery
time, Trec = 3.5 s. In C) and D), a zoomed graph
for two RF pulses is shown in the black rectangles. The phase accumulation due
to the off-resonance of the RF pulses is taken into account (D).
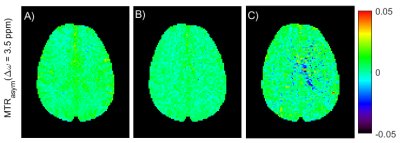
MTRasym maps at 3.5 ppm for the APTw_3T_001
(A), APTw_3T_002 (B) and APTw_3T_003 (C) protocols. Protocols were run with
repeated acquisitions (3 repetitions) at the offsets of interest and a dummy
scan at the beginning.
-
Assessment of acquisition strategies of CEST MR fingerprinting pH imaging using Cramer-Rao Bound algorithm
Jie Liu1, Hui Liu2, Qi Liu2, Jian Xu2, Hairong Zheng1, and Yin Wu1
1Paul C. Lauterbur Research Center for Biomedical Imaging, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2United Imaging Healthcare America, Houston, TX, United States
We designed
a Cramer-Rao bound based metric, and demonstrated its ability in evaluating the
performance of acquisition strategies of CEST-MRF imaging in pH quantification.
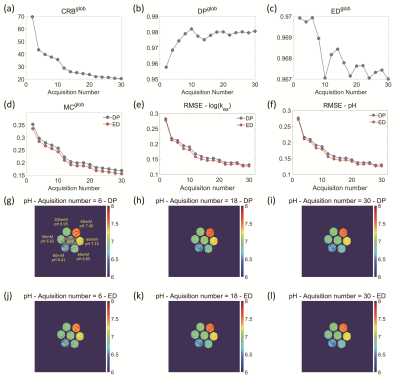
Figure 3. (a-c) Values of CRB-, DP-, ED-based indexes with
varied acquisition number of 2-30; (d) RMSE of pH in MC simulation study using
both DP- and ED-based matching methods; RMSE of (e) log(kex) and (f)
respective pH values of the phantoms; the CEST-MRF measured pH maps in the
phantom study using both (g-i) DP- and (j-l) ED-based matching methods for representative
acquisition numbers of (g, j) 6, (h, k) 18 and (i, l) 30.
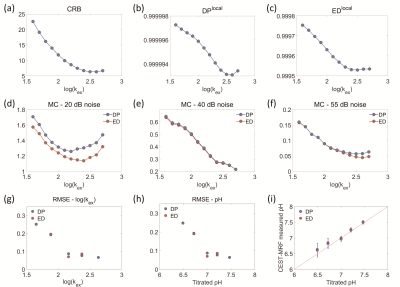
Figure 1. (a-c) Alterations of CRB-,
DP-, ED-based metrics with exchange
rates; RMSE of pH quantification
in MC simulation study using both DP- and ED-based matching methods under noise
levels of (d )20, (e) 40 and (f) 55 dB; RMSE of (g) log(kex) and (h)
pH of the phantom; (i) correlation of the measured and the titrated pH values
for vials with the same concentration of 60mM.
-
A comparison of several tumor endogenous pH mapping methods using CEST at 11.7T
Ying Liu1,2, Botao Zhao1,2, and Xiao-Yong Zhang1,2
1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 2Key Laboratory of Computational Neuroscience and Brain-Inspired Intelligence (Fudan University), Ministry of Education, Shanghai, China
We compared several tumor endogenous
pH mapping methods using CEST at 11.7T. All of these pH-weighted contrasts showed a pH correlation, while the pH enhanced and APT methods were not
concentration-independent and the sensitivity of AACID was affected by the weak
amine signals at 2.7ppm.
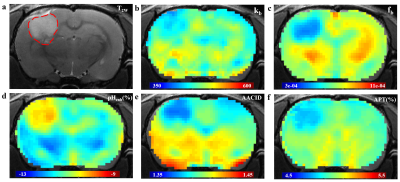
Fig. 2. Representative examples of structural
image and CEST maps of one mouse brain. (a)T2W image with tumor
region delineated by the red dotted line. (b), (c) Representative proton
exchange rate and labile proton ratio maps. (d), (e), (f) pH weighted contrasts
calculated using pH enhanced method, AACID method and amide proton transfer
(APT) MRI.
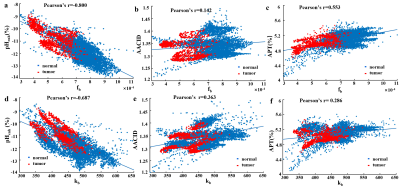
Fig. 3. Association between labile
proton
ratio (fb) , chemical exchange rate (kb) values and AACID (a) (d)
,pHenh
(b)
(e),
APT(c)
(f) values
of
all voxels in 6 mice brain from tumors (red points) and normal tissue (blue
points).
-
Whole-brain amide CEST at 3T with a steady-state radial MRI acquisition
Ran Sui1,2,3, Lin Chen1,2, Yuguo Li1,2, Jianpan Huang4, Kannie W.Y Chan2,4, Xiang Xu5, Peter C.M.van Zijl1,2, and Jiadi Xu1,2
1F.M. Kirby Research Center for Functional Brain Imagin, Kennedy Krieger Institute, Baltimore, MD, United States, 2Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Department of Biomedical Engineering, Johns Hopkins University, Baltimore, MD, United States, 4Department of Biomedical Engineering, City University of Hong Kong, Hong Kong, China, 5BioMedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Acquire whole-brain amide CEST mapping at 3T MRI by using steady-state radial sampling CEST with PROPELLOR-based sampling
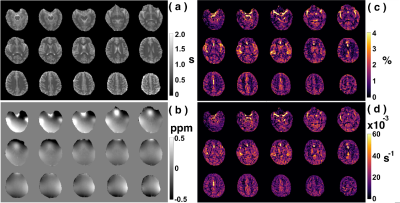
Figure 3: Typical multi-slice human brain images acquired with starCEST at 3T. (a) T1 maps by look-locker sequence; (b) B0 maps acquired with dual-echo sequence, The (c) amideCEST maps (ΔZamide) and the corresponding (d) apparent relaxation rate (Ramide) maps extracted with the PLOF method by including the B0 and T1 maps, respectively.
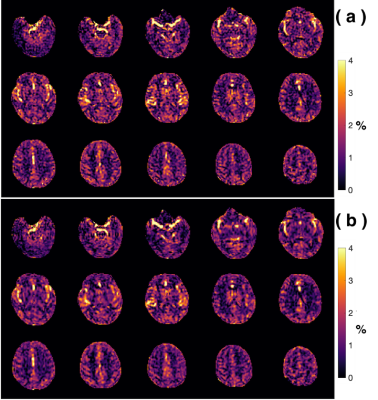
Comparison of the amideCEST maps without and with MLSVD post-processing. (a) The original amide CEST maps extracted using PLOF without MLSVD denoising. (b) MLSVD denoising applied with truncation numbers 48, 48 and 10.
-
Investigating MT and CEST Characteristics of DU145 Prostate Tumour Xenografts in Relation to Radiation Treatment Response
Leedan Murray1, Wilfred W. Lam1, and Greg J. Stanisz1,2
1Physical Sciences, Sunnybrook Research Institute, Toronto, ON, Canada, 2University of Toronto, Toronto, ON, Canada
MT and CEST characteristics of DU145 prostate tumour
xenografts with known responses to radiation were investigated. Significant changes between responses
were mostly observed in the necrotic/apoptotic regions of the tumour and were
most evident in the qMT parameters.
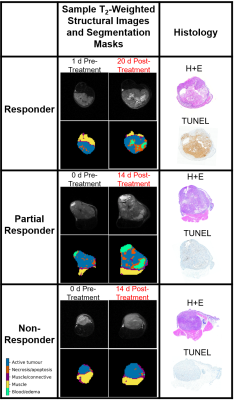
Figure 1: Representative
segmentation masks, T2-weighted images and histology (H+E and TUNEL
stains) for each category of response to radiation treatment (responder, partial
responder, non-responder).
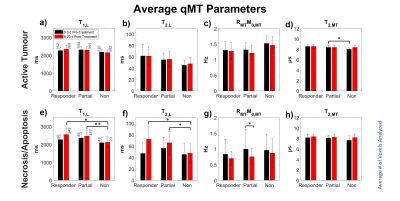
Figure 4: Average
estimated qMT parameters by treatment response of (a-d) active and (e-h)
necrotic/apoptotic regions. Free parameters were: T1 and T2
of the water pool (T1,L and T2,L respectively), the exchange rate of
magnetization from the MT pool to the water pool (RMT), original
magnetization of the MT pool relative to the water pool (M0,MT), and
T2 of the MT pool (T2,MT). *p < 0.05. **p < 0.01.
-
An ASL and CrCEST combined protocol at 3T in the Study of Metabolic and Perfusion Changes Post Revascularization in Peripheral Arterial Disease
Helen Sporkin1, Toral Patel2, Christopher Schumann2, Christopher Kramer2,3, and Craig Meyer1,3
1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Cardiovascular Medicine, University of Virginia, Charlottesville, VA, United States, 3Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, United States
Our preliminary findings in six patients show success in imaging PAD patients with CrCEST and exercise-induced hyperemia ASL in the same protocol. Further recruitment is necessary to establish findings linking perfusion, metabolism, and function.
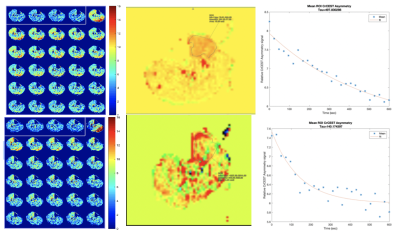
Figure 1: (Top row) CrCEST time course imaging (left) ,and ASL (middle), and overall CrCEST decay (right) in a patient with an ABI of 0.68 prior to endovascular revascularization.(Bottom row) CrCEST time course imaging (left) , ASL (middle), and overall CrCEST decay (right) CrCEST time course imaging and ASL in the same patient 6 months after revascularization, showing changes in both metabolism and perfusion.
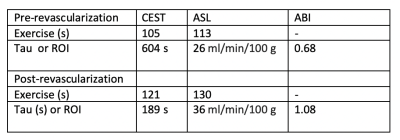
Table 1: CEST and ASL evaluation pre and post revascularization in patient 1. ASL and CEST measurements are reported as a mean of the muscle ROI in which the greatest perfusion was seen in the ASL images.
