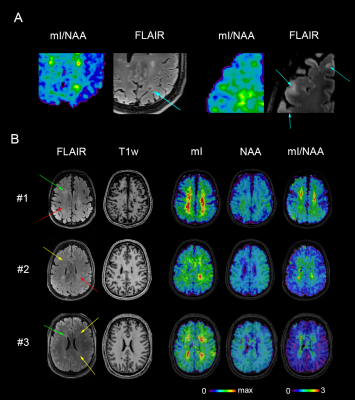
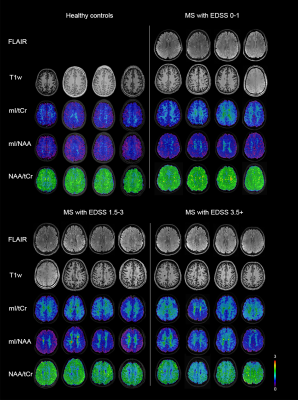
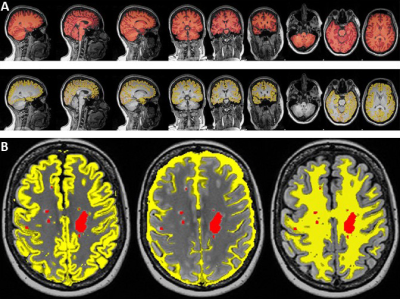
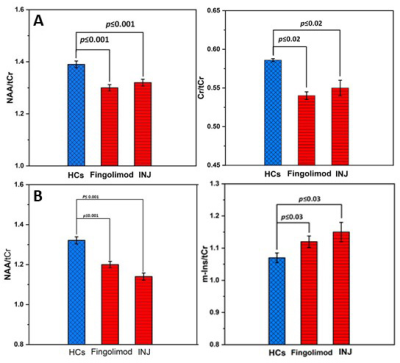
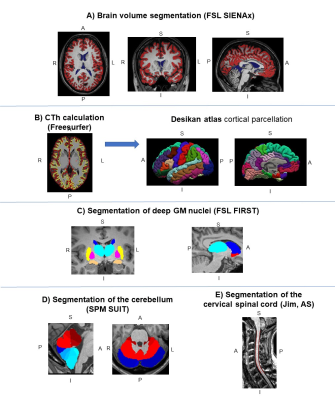
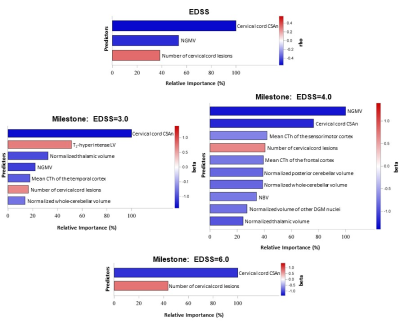
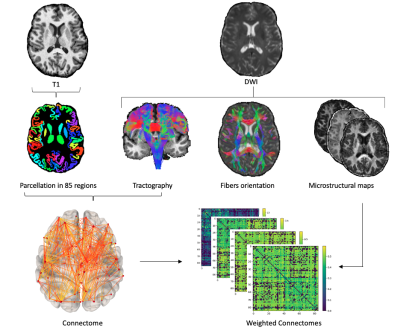
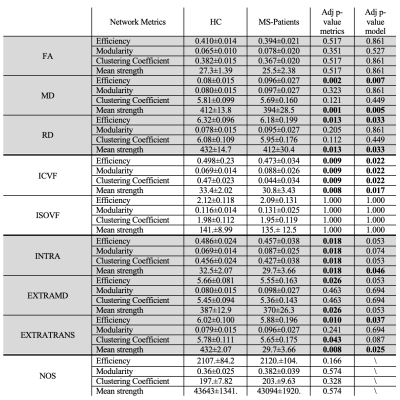
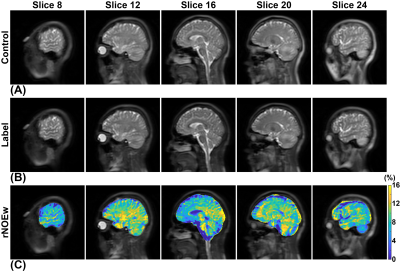
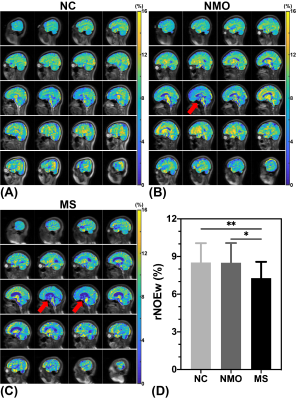
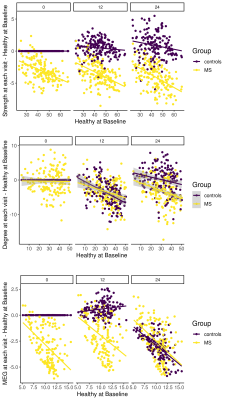
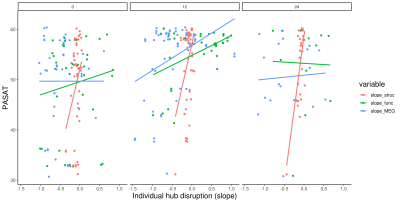
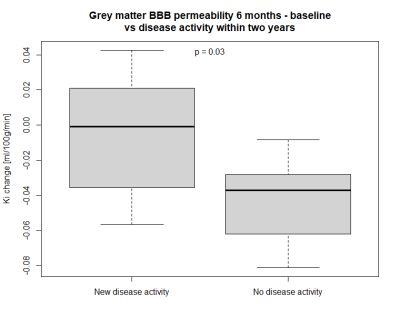
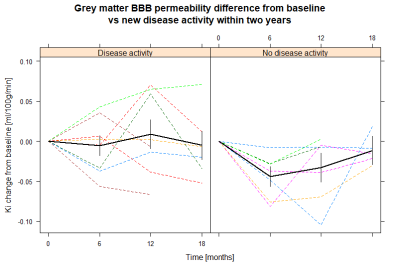
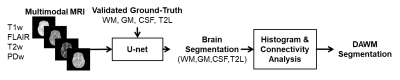
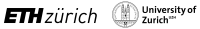-
Paramagnetic Rim Lesions in MS are characterized by heterogeneous damage and inflammatory activity: a combined T1 relaxometry-diffusion study
Muhamed Barakovic1,2,3, Riccardo Galbusera1,2,3, Reza Rahmanzadeh1,2,3, Matthias Weigel1,2,3, Po-Jui Lu1,2,3, Erik Bahn4, Simona Schiavi5, Alessandro Daducci5, Pascal Sati6,7, Pietro Maggi8,9, Ludwig Kappos2,3, Jens Kuhle2,3, Laura Gaetano10, Stefano Magon11, and Cristina Granziera1,2,3
1Translational Imaging in Neurology (ThINk) Basel, University Hospital Basel and University of Basel, Basel, Switzerland, 2Neurologic Clinic and Policlinic, University Hospital Basel and University of Basel, Basel, Switzerland, 3Research Center for Clinical Neuroimmunology and Neuroscience (RC2NB) Basel, University Hospital Basel and University of Basel, Basel, Switzerland, 4Institute of Neuropathology, University Medical Center, Göttingen, Germany, 5Department of Computer Science, University of Verona, Verona, Italy, 6Translational Neuroradiology Section, National Institute of Neurological Disorders and Stroke, Bethesda, MD, United States, 7Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 8Department of Neurology, Lausanne University Hospital, Lausanne, Switzerland, 9Cliniques universitaires Saint Luc, Université catholique de Louvain, Louvain, Belgium, 10F. Hoffmann-La Roche Ltd., Basel, Switzerland, 11Pharmaceutical Research and Early Development, Roche Innovation Center Basel F. Hoffmann-La Roche Ltd., Basel, Switzerland
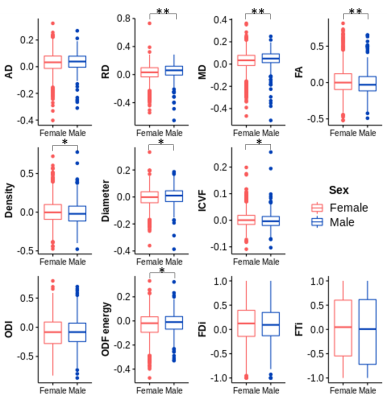
Paramagnetic rim lesions (PRL) are characterized by heterogeneous damage in multiple sclerosis (MS) patients. In this study we acquired in vivo, postmortem and histopathology with the aim to further characterize the microstructural properties of these lesions.
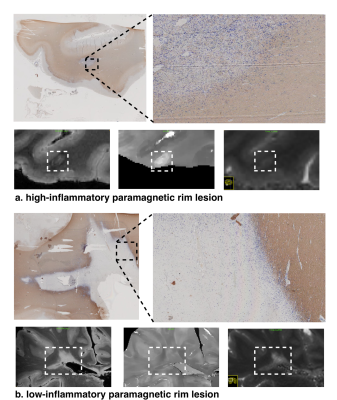
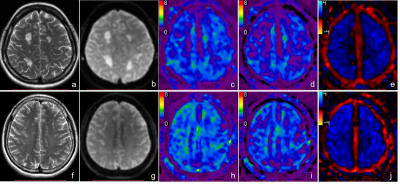
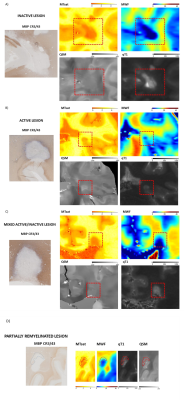
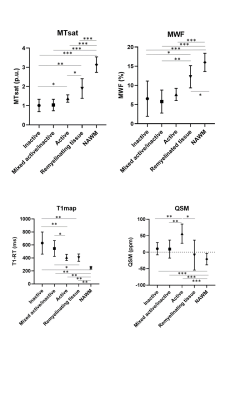
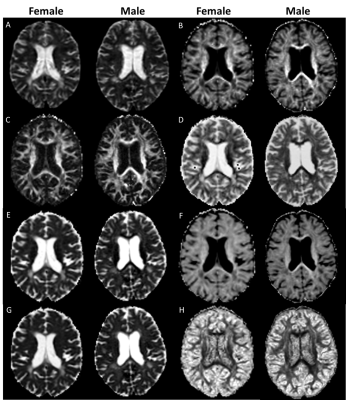
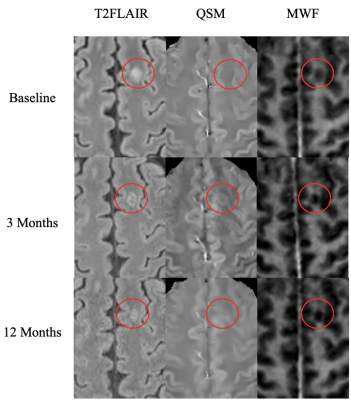
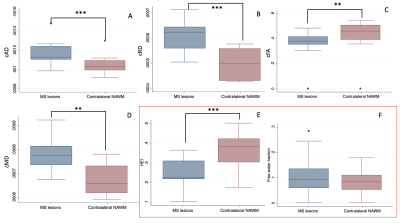
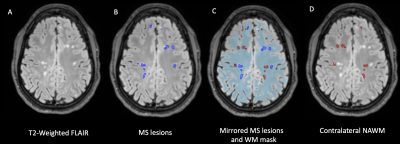
Figure 1: Post mortem images showing an exemplary high-inflammatory PRL (a.) and a low-inflammatory PRL (b). (top) Double immunohistochemistry of myelin basic-protein (MBP) (brown) with CR3/43 (MHC II, (blue) in an exemplary highly inflamed PRL (box, a.) and low-inflammatory PRL (box, b.) On the bottom, 3D EPI, QSM derived from 3D EPI and qT1 derived from MP2RAGE.
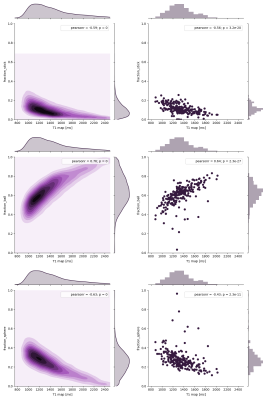
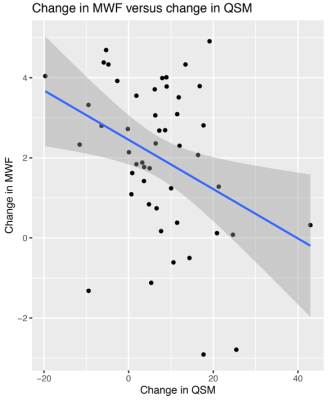
Figure 2. Joint plot showing the relationship between qT1 and diffusion parameters estimated from multi-shell diffusion MRI. Left: Voxel-wise joint plot with kernel density estimate (KDE) Right: Lesion-wise plot showing the relationship between qT1 and diffusion parameters using a scatter plot. First row: stick fraction, representing the intra-cellular component. Second row: ball fraction, representing the extra-cellular unrestricted component. Third row: sphere fraction, representing the extra-cellular restricted component.
-
Deep Multiple Sclerosis Lesion Segmentation with Anatomical Convolution and Lesion-wise Loss
Hang Zhang1, Jinwei Zhang1, Pascal Spincemaille1, Thanh D. Nguyen1, and Yi Wang1
1Cornell University, New York, NY, United States
We propose an anatomical convolutional module and lesion-wise sphere loss for improving MS lesion segmentation.
Figure 1. Example Illustration of the
proposed anatomical coordinates with one slice from the axial direction.
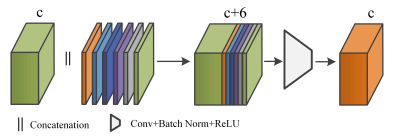
Figure 2. The computed anatomical
coordinates are concatenated with the incoming feature tensor through channel
dimension, followed by standard convolution, batch normalization, and ReLU
activation for feature fusion. c is the number of channels in the feature
tensor.
-
Characterization of multiple sclerosis lesion types with texture analysis of advanced and conventional MRI
Zahra Hosseinpour1, Olayinka Oladosu2, Mahshid Soleymani1, G Bruce Pike2, and Yunyan Zhang2
1Biomedical Engineering program, Schulish School of Engineering, University of Calgary, Calgary, AB, Canada, 2Department of Radiology and Clinical Neurosciences, Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada
Texture analysis of MRI detected several tissue types
in multiple sclerosis (MS). It identified de- and re-myelination in MS
postmortem and differentiated the highly demyelinated and potentially remyelinated
lesions in MS patients.
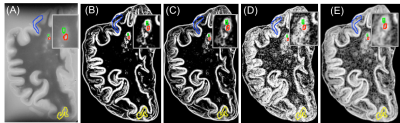
Figure 1. Postmortem T2-MRI and calculated
maps. The shown are T2 (A), T2-Contrast (B), T2-Dissimilarity (C), T2-Entropy
(D): all from average GLCM, and T2-entropy filter (E). Different colors
demonstrate different lesion types: red: WML, green: remyelinated WML, blue:
type IV cortical lesion, yellow: type III cortical lesion.
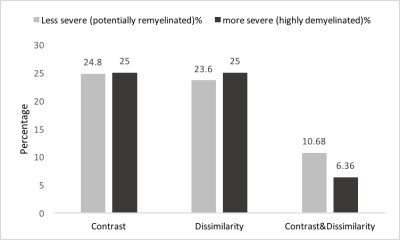
Figure 4) In-vivo lesion severity. 25th
percentile of contrast and both contrast and dissimilarity were used to detect
the less severe (potentially remyelinated) lesions. And 75th
percentile of the same parameters were applied to have more severe lesions (highly
demyelinated and axonal damaged). We found that most of the patients have both
types of lesions and the percentage of the less severe lesions is in the range
of reported remyelination percentage in MS.
-
MRI reveals inflammation-mediated demyelination in a cuprizone toxin model of multiple sclerosis
Stephen T. Vito1, Kai H. Barck2, Rohan S. Virgincar2, Amy Easton1, Robby M. Weimer2, Tracy J. Yuen1, and Luke Xie2
1Neuroscience, Genentech, South San Francisco, CA, United States, 2Biomedical Imaging, Genentech, South San Francisco, CA, United States
MRI detected inflammation-mediated demyelination in the cuprizone model. Voxel-based analysis revealed significant lesions in the medial splenium, lateral genu, and cerebellar nuclei. T2 reflected inflammation (microglia and astrocytes) while MWF and gRatio suggested demyelination.
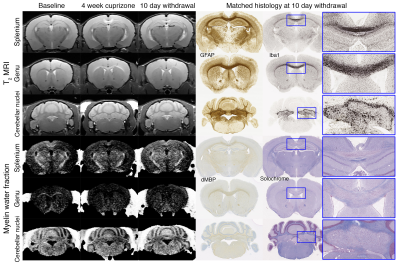
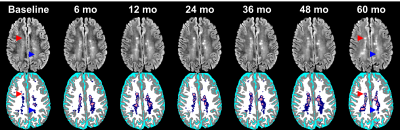
Fig. 1. Longitudinal MRI of brain lesions at baseline, 4-week cuprizone, and 10-day withdrawal (n=12). Lesions are observed in the splenium, genu, and cerebellar nuclei in T2-weighted and myelin water fraction images. Example histology of the same animals are shown. Inserts show lesion areas at 5× magnification. GFAP~astrocytes, Iba1~microglia, dMBP~denatured myelin basic protein, and solochrome~myelin.
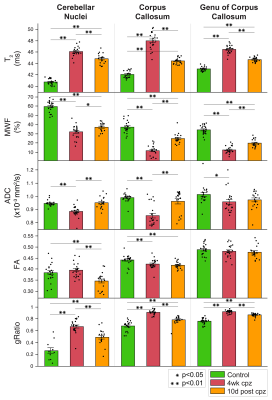
Fig. 2. Bar plots for longitudinal MRI. Error bars show standard error about the mean. Sample size: n=12 at baseline, 4-week cuprizone, and 10-day withdrawal. MWF=myelin water fraction, ADC=apparent diffusion coefficient, and FA=fractional anisotropy. One-way ANOVA and post-hoc t-tests were performed between baseline control, 4-week cuprizone, and 10-day withdrawal groups (*p<0.05, **p<0.01).
-
Longitudinal automated assessment of paramagnetic rim lesions in multiple sclerosis using RimNet
Maxence Wynen1, Francesco La Rosa2,3,4, Amina Sellimi5, Germán Barquero2,3,4, Gaetano Perrotta6, Valentina Lolli7, Vincent Van Pesch5, Cristina Granziera8,9, Tobias Kober10, Pascal Sati11,12, Benoît Macq13, Daniel S. Reich11, Martina Absinta11,14, Meritxell Bach Cuadra2,3,4, and Pietro Maggi5,15
1Ecole Polytechnique de Louvain, Université Catholique de Louvain, Louvain-la-Neuve, Belgium, 2Signal Processing Laboratory (LTS5), École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 3Medical Image Analysis Laboratory, Center for Biomedical Imaging (CIBM), University of Lausanne, Lausanne, Switzerland, 4Radiology Department, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 5Department of Neurology, Cliniques Universitaires Saint-Luc, Université Catholique de Louvain, Brussels, Belgium, 6Department of Neurology, Erasme University Hospital, Université Libre de Bruxelles, Brussels, Belgium, 7Department of Radiology, Erasme University Hospital, Université Libre de Bruxelles, Brussels, Belgium, 8Neurologic Clinic and Policlinic, Departments of Medicine, Clinical Research and Biomedical Engineering, University Hospital Basel and University of Basel, Basel, Switzerland, 9Translational Imaging in Neurology (ThINk) Basel, Department of Medicine and Biomedical Engineering, University Hospital Basel and University of Basel, Basel, Switzerland, 10Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 11Translational Neuroradiology Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, United States, 12Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 13ICTEAM Institute, Université catholique de Louvain, Louvain-la-Neuve, Belgium, 14Department of Neurology, Johns Hopkins University, Baltimore, MD, United States, 15Department of Neurology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland
RimNet, a deep-learning-based segmentation
algorithm for chronic active multiple sclerosis lesions, is effective for unseen
MRI data acquired on a scanner from a different vendor, and makes consistent
predictions on longitudinal data.
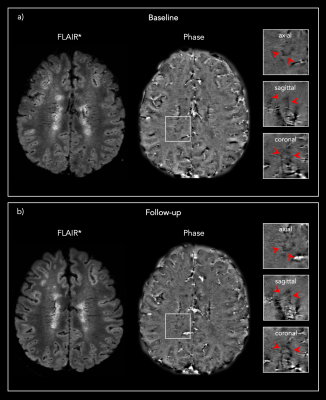
Figure 1: Representative 3D FLAIR* and Phase images from one
PMS patient showing the same PRL a) at baseline and b) at the 13 months post-DMT
follow-up.
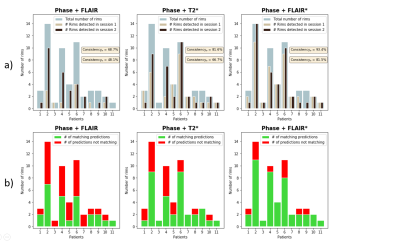
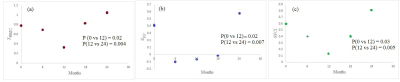
Figure 3: Summary of the lesion-wise results for each patient with paramagnetic rim lesions (n=11 of 13 total patients studied, 85%). a) Number of rims detected in session 1 (baseline) and session 2 (follow-up) for each patient, overlaid on the total number of rims adjudicated in the consensus manual reading. b) Number of matching predictions between baseline and follow-up. Abbreviations: Consistencyp, probability-based consistency; Consistencyb, binary-based consistency.
-
Accelerating Quantification of Myelin Water Fraction with Nonlocal Low-Rank Tensor in the Feature Domain
Quan Chen1, Huajun She1, and Yiping P. Du1
1School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China
A Feature domain nonlocal Low-Rank Tensor based (FnLRT)
algorithm is proposed to accelerate the quantification of myelin water
content. The FnLRT algorithm provides the potential to obtain the whole
brain MWF mapping in 1 minute.
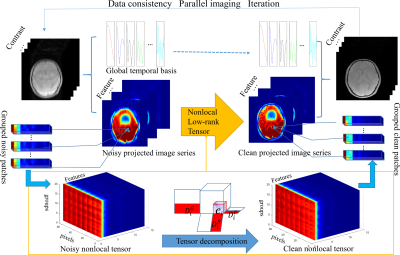
Figure 1. The flowchart of the
FnLRT algorithm. The artifacts in the features are substantially reduced.
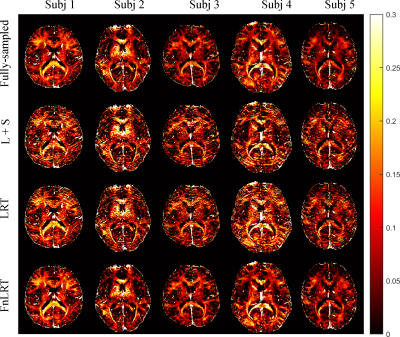
Figure 3. The MWF maps of 5 representative subjects obtained from the L+S, LRT and
FnLRT reconstructions (R = 6).
-
Beyond the Mean: Myelin Heterogeneity Index as a Sensitive Metric for Assessing Myelin Damage in Multiple Sclerosis
Poljanka Johnson1, Irene M Vavasour2, Shawna Abel1, Lisa E Lee3, Stephen Ristow1, Cornelia Laule4, Roger Tam2, David K.B. Li2, Nathalie Ackermans1, Alice Schabas1, Jillian Chan1, Helen Cross1, Ana-Luiza Sayao1, Virginia Devonshire1, Robert Carruthers1, Anthony Traboulsee3, and Shannon Kolind5
1Medicine, University of British Columbia, Vancouver, BC, Canada, 2Radiology, University of British Columbia, Vancouver, BC, Canada, 3University of British Columbia, Vancouver, BC, Canada, 4Radiology, Physics and Astronomy, Pathology, University of British Columbia, Vancouver, BC, Canada, 5Medicine, Physics & Astronomy, University of British Columbia, Vancouver, BC, Canada
This study investigates a potential myelin related metric for discriminating between MS subtypes. This study also demonstrates that normal appearing white matter in MS shows increased myelin damage in individuals with worse disability.
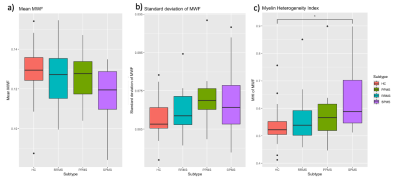
The myelin water fraction (MWF) a) mean, b) standard deviation, and c) coefficient of variation (or myelin heterogeneity index, MHI) of the normal appearing white matter in healthy controls (HC), relapsing remitting MS (RRMS), primary progressive MS (PPMS), and secondary progressive MS (SPMS). A one-way ANOVA test with Tukey correction showed that the MHI of SPMS was higher than HC (p=0.01) and trended towards higher than RRMS (p=0.065).
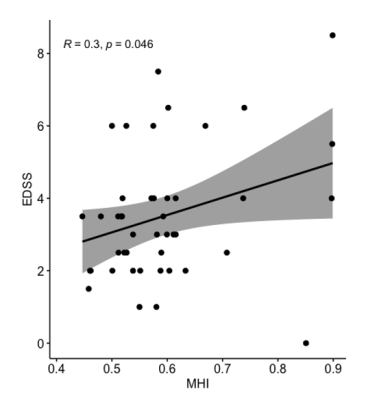
The myelin heterogeneity index (MHI = MWF standard deviation/MWF mean) of all MS patients was correlated with Expanded Disability Status Scale (EDSS) (p = 0.046, R = 0.3) using a Pearson correlation. Shaded area is 95% confidence interval.
-
Application of an exponential recovery model to multiparametric 3D MRI to characterize the evolution of active lesions in Multiple Sclerosis
Lucas Soustelle1,2, Andreea Hertanu1,2, Arnaud Le Troter1,2, Soraya Gherib1,2, Samira Mchinda1,2, Patrick Viout1,2, Lauriane Pini1,2, Claire Costes1,2, Sylviane Confort-Gouny1,2, Adil Maarouf1,2,3, Bertrand Audoin1,2,3, Audrey Rico1,2,3, Clémence Boutière1,2,3, Maxime Guye1,2, Jean-Philippe Ranjeva1,2, Gopal Varma4, David C. Alsop4, Jean Pelletier1,2,3, Olivier M. Girard1,2, and Guillaume Duhamel1,2
1Aix Marseille Univ, CNRS, CRMBM, Marseille, France, 2APHM, Hôpital Universitaire Timone, CEMEREM, Marseille, France, 3APHM, Hôpital Universitaire Timone, Service de neurologie, Marseille, France, 4Division of MR Research, Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States
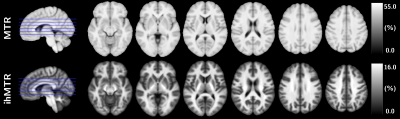
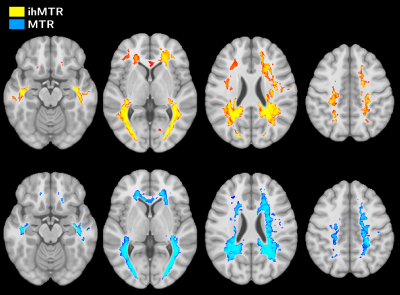
Active
MS lesions were evaluated over a 12-month follow-up study by modelling the
signal evolution of ihMT, conventional MT, DTI and T1w data. Results show that MR
metrics in lesions recover at different rates, emphasizing different sensitivity
to MS physiopathology mechanisms.
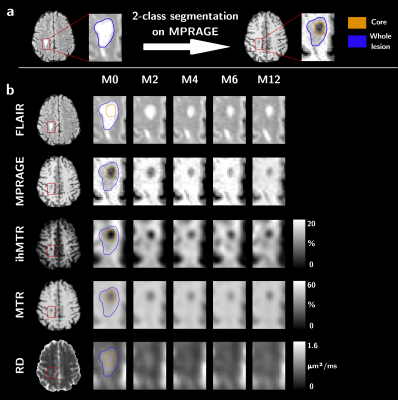
Figure 1: Sketch of the sub-segmentation processing of the lesion into core
and edge regions based on the T1w contrast and from the manually drawn mask on
the FLAIR image (a), and illustrative depiction of the evolution of MR
contrasts over time in an active lesion (b).
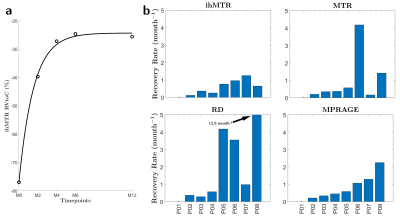
Figure 3: Example of an RVtoC curve adjustment over an ihMTR lesion in the
core region (a), and boxplots of the average recovery rates per patients of
ihMTR, MTR, RD and MPRAGE (b). Note that patient P01 was not included as the
dynamic of its two lesions could not be resolved by the fitting model for both
ihMTR and RD (ρ²<0.75).
-
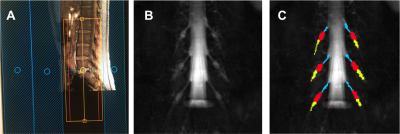
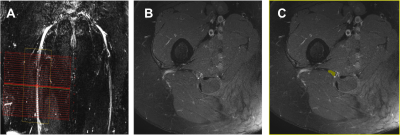
High-b Diffusivity of MS Lesion in Cervical Spinal Cord using Ultrahigh-b DWI (UHb-DWI)
Kyle Jeong1, Lubdha Shah2, You-Jung Lee1, Bijaya Thapa1, Nabraj Sapkota1, Erica Bisson3, Noel Carlson4, Eun-Kee Jeong2, and John Rose5
1Utah Center for Advanced Imaging Research, University of Utah, Salt Lake City, UT, United States, 2Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States, 3Neurosurgery, University of Utah, Salt Lake City, UT, United States, 4GRECC, Veterans Affairs, Salt Lake City, UT, United States, 5Neuroimmunology and Neurovirology Division, University of Utah, Salt Lake City, UT, United States
The main objective of this study was to present UHb-rDWI as a potential powerful tool in quantitatively characterizing MS CSC. We investigate UHb-rDWI signal in white matter tracts of the CSC and compare quantitative values between healthy control WM with both MS NAWM and WM lesions.
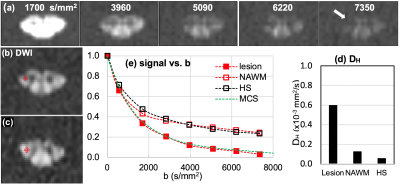
Fig. 1. MS1: (a) DWIs of b = 1700 ~ 7350 s/mm2 of the lesion slice and (b) ROIs at lesion and NAWM slice, 1.5 cm above the lesion toward the brain, and (c) signal-b curves of the recently-active lesion (red square) and NAWM (red circle), and averaged data (hollow square) at the corticospinal tract of seven healthy subjects, and (d) mean values of high-b diffusion coefficient DH at the lesion, NAWM, and the healthy CSCs at the corticospinal tract. Green dotted line in (b) indicates the MCS data with 30 % demyelination in lesion.
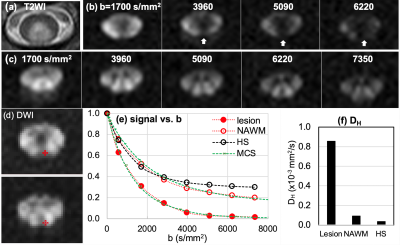
Fig. 2. MS2: (a) T2WI, (b, c) DWIs of b = 1700 ~ 7350 s/mm2 of the lesion slice and NAWM slice, 1.5 cm below the lesion away from the brain, (d) ROIs on lesion (top) and NAWM (bottom), (e) signal-b curves of the recently-active lesion, NAWM, and averaged data at the posterior column of 7 HS, and (f) mean values of high-b diffusion coefficient DH at the lesion, NAWM, and the healthy CSCs at the corticospinal tract. White vertical arrows in (a) indicate lesion with rapidly decaying signal. Green dotted lines in (e) indicate the MCS data with 40 % and 15 % demyelination in lesion and NAWM, respectively.
-
Brain Ultrashort T2 Component imaging using a STAIR Prepared Dual-Echo UTE Sequence with Complex Echo Subtraction
Ya-Jun Ma1, Hyungseok Jang1, Zhao Wei1, Mei Wu1, Saeed Jerban1, Eric Y Chang1,2, Jody Corey-Bloom1, Graeme M Bydder1, and Jiang Du1
1UC San Diego, San Diego, CA, United States, 2VA Health system, San Diego, CA, United States
UltraShort T2
Proton Fraction (USPF) reduction in multiple
sclerosis (MS) lesions
suggests that the proposed STAIR-dUTE-ES technique has potential for
evaluation of demyelination and remyelination in the diagnosis and treatment of
patients with MS.
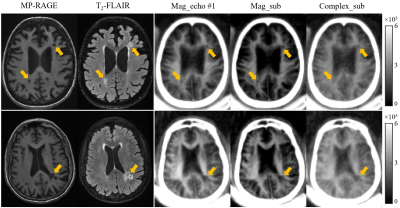
Figure 3 Selective clinical MP-RAGE (first
column), T2-FLAIR (second column) and STAIR-dUTE (last three
columns) images of two representative patients with MS (first row: a 49-year-old
female; second row: a 69-year-old female). MS lesions appeared hypointense on
the MP-RAGE image and hyperintense on the T2-FLAIR image as indicated
by the yellow arrows. These lesions also show signal loss on the magnitude images in the first echo images (third
column), magnitude echo subtracted images (fourth column) and complex echo
subtracted images (last column) using the STAIR-dUTE sequence.
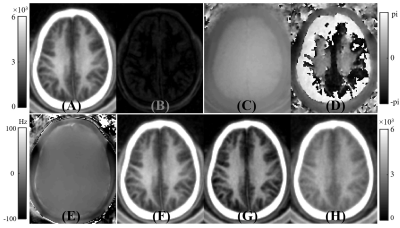
Figure 2 A volunteer study showing the generation of ultrashort T2 signals with the methods used in this study. The first row shows magnitude
images for the first echo (A) and the second echo (B), as well as the corresponding
phase images for the first echo (C) and second echo (D). Panel E shows the ΔB0
field map used for the complex ES. Magnitude of the first echo (F), magnitude
echo subtracted (G), and complex echo subtracted (H) images obtained with the STAIR-dUTE
sequence are shown. The magnitude of the first echo image (A) is displayed
again in (F) for closer comparison with (G) and (H).
-
Myelin Water Imaging Demonstrates Myelin Loss in Multiple Sclerosis Normal Appearing White Matter over Two Years
Irene Margaret Vavasour1, Poljanka Johnson2, Shawna Abel3, Stephen Ristow3, Jared Splinter3, Cornelia Laule1,4,5,6, Roger Tam1, David KB Li1, Nathalie Ackermans3, Alice J Schabas3, Jillian Chan3, Helen Cross3, Ana-Luiza Sayao3, Virginia Devonshire3, Robert Carruthers3, Anthony Traboulsee3, and Shannon H Kolind1,3,4,6
1Radiology, University of British Columbia, Vancouver, BC, Canada, 2Neuroscience, University of British Columbia, Vancouver, BC, Canada, 3Medicine, University of British Columbia, Vancouver, BC, Canada, 4Physics and Astronomy, University of British Columbia, Vancouver, BC, Canada, 5Pathology and Laboratory Medicine, University of British Columbia, Vancouver, BC, Canada, 6International Collaboration on Repair Discoveries (ICORD), University of British Columbia, Vancouver, BC, Canada
Using multi-echo T2 relaxation, a decrease in mean myelin
water fraction over two years was detected in the normal appearing white matter
of relapsing-remitting and progressive multiple sclerosis participants.
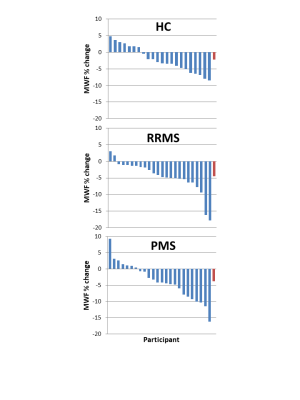
Figure 4: Percentage change over 2 years in mean myelin water
fraction (MWF) for each
participant
divided
into participant subtype (healthy control (HC), relapsing remitting multiple
sclerosis (RRMS), progressive multiple sclerosis (PMS)).
The last bar (in red) is the mean over the participants.
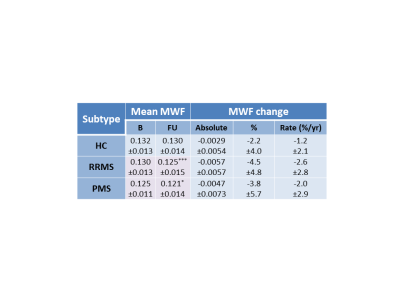
Figure 2: Mean myelin water fraction (±standard
deviation) at baseline (B) and follow-up (FU),
absolute change in MWF over 2 years, percent MWF change over 2 years (%) and annual rate of MWF percent change divided into
participant subtype
(healthy control (HC), relapsing remitting multiple sclerosis (RRMS),
progressive multiple sclerosis (PMS)). Volumes are taken at baseline. Significant differences between baseline
and follow-up are denoted in pink with *p<0.01, ***p<0.0001.
-
Sub-second Accurate Myelin Water Fraction Reconstruction with UNET
Jeremy Kim1,2, Thanh Nguyen2, Jinwei Zhang2, and Yi Wang2
1Stanford University, New York, NY, United States, 2Weill Cornell, New York, NY, United States
We developed UNET neural network for sub-second, accurate, and reproducible extraction of myelin water fraction map from FAST-T2 multi-echo T2 decay data.
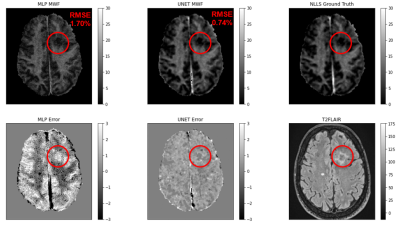
Figure 1: Representative case showing the UNET’s prediction against
the ANN’s prediction
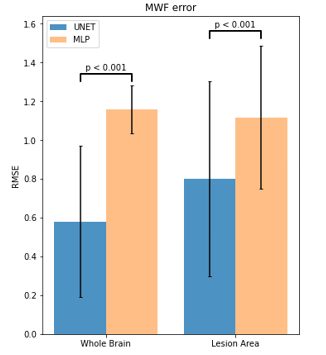
Figure 2: RMSE for UNET and ANN Predictions
-
Quantitative multi-modal MRI shows correlations between lesion iron deposition and neuro-axonal density in progressive multiple sclerosis
Sara Collorone1, Marco Battiston1, Ferran Prados 1,2,3, Alberto Calvi1, Baris Kanber4, Francesco Grussu1,5, Marios Yiannakas1, Carmen Tur1,6, Rebecca Samson1, Olga Ciccarelli1,7, and Claudia A.M. Gandini Wheeler-Kingshott1,8,9
1NMR Research Unit, Queen Square MS Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, Faculty of Brain Sciences, University College London (UCL), London, United Kingdom, 2Centre for Medical Image Computing (CMIC), Department of Medical Physics and Biomedical Engineering, University College London (UCL), London, United Kingdom, 3Universitat Oberta de Catalunya, Barcelona, Spain, 4Centre for Medical Image Computing (CMIC), Department of Medical Physics and Biomedical Engineering, University College London(UCL), London, United Kingdom, 5Radiomics Group, Vall d’Hebron Institute of Oncology, Vall d’Hebron Barcelona Hospital Campus, Barcelona, Spain, 6Multiple Sclerosis Centre of Catalonia (Cemcat), Vall d’Hebron Institute of Research, Vall d’Hebron Barcelona Hospital Campus, Barcelona, Spain, 7University College London Hospitals, Biomedical Research Centre, National Institute for Health Research, London, United Kingdom, 8Department of Brain & Behavioural Sciences, University of Pavia, Pavia, Italy, 9Brain Connectivity Center Research Department, IRCCS Mondino Foundation, Pavia, Italy
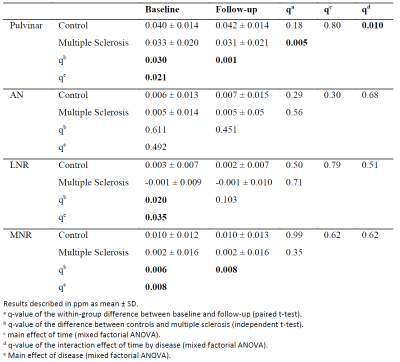
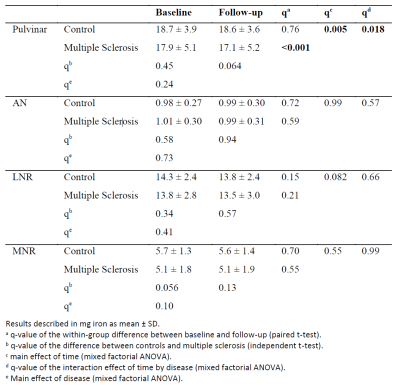
In progressive multiple sclerosis, iron deposition in the lesions correlates with axonal loss in the lesions and cortical grey matter. Both iron deposition and axonal loss in lesions correlate with cognitive disability.
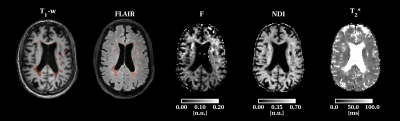
Figure 4: Example of T1 and 3DFLAIR images, F, NDI and T2* maps in a patient. F and NDI are low in the white matter lesions while T2* values are increased.
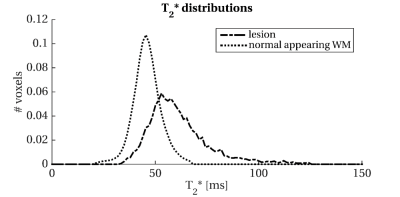
Figure 5: Histogram of T2* distribution in the normal-appearing white matter and white matter lesions. Abbreviations: WM: white matter
-
A Comparison of Brain Iron Accumulation Patterns Between Subtypes of Multiple Sclerosis
Aly Khalifa1 and Michael D Noseworthy2,3
1Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 2Electrical and Computer Engineering, McMaster University, Hamilton, ON, Canada, 3School of Biomedical Engineering, McMaster University, Hamilton, ON, Canada
Susceptibility weighted imaging (SWI) was used to correlate brain regional iron levels with disease severity in the four subtypes of multiple sclerosis (MS).
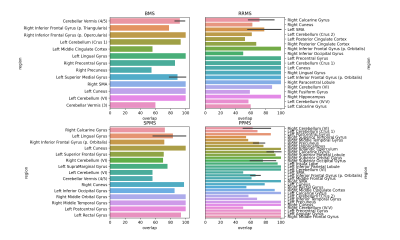
Figure 3. The mean percent overlap of clusters with anatomical regions labeled in the N27 Atlas, by MS subtype. The percent overlap is defined as the number of voxels within a cluster that overlap with an anatomical region, divided by the total number of number of voxels in the cluster. Error bars represent the 95% confidence interval, calculated only for regions with more than one cluster.
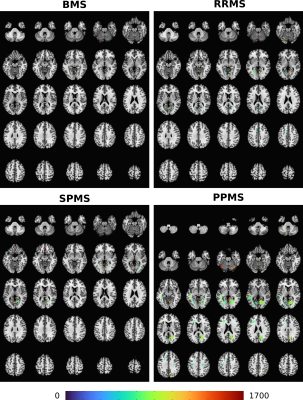
Figure 2. Montages of clusters identified by thresholding the mean difference in SWI intensity between each MS cohort (BMS, RRMS, SPMS, PPMS) and the control group. The clusters are overlain on to the N27 atlas. The colourmap corresponds to the square root of the mean sum of squares due to treatment effect. A significance threshold of p=0.001 was used.
-
MS-Voter: Learning Where to Vote for Confluent Multiple Sclerosis Lesion Separation
Hang Zhang1, Jinwei Zhang1, Junghun Cho1, Susan A. Gauthier1, Pascal Spincemaille1, Thanh D. Nguyen1, and Yi Wang1
1Cornell University, New York, NY, United States
A machine learning technique is described for
separating confluent lesions in multiple sclerosis using Hough voting and
K-means clustering.
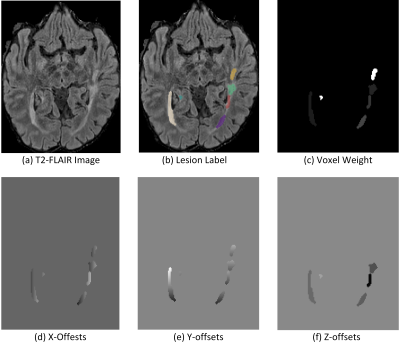
Figure 1. Example Illustration of how to
compute lesion offsets and voxel weight based on ground-truth lesion labels.
Individual lesions are marked by masks of different colors.
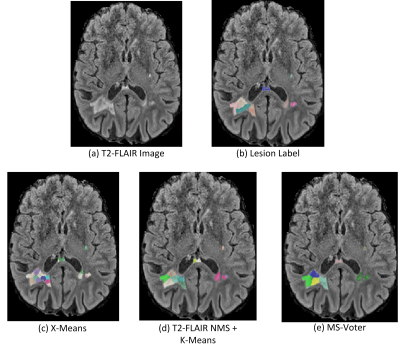
Figure 2. Qualitative comparison between
baseline methods and our proposed MS-Voter.
-
Increasing age is independently associated with higher free water in non-active MS brain - A multi-compartment analysis using FAST-T2
Liangdong Zhou1, Yi Li1, Xiuyuan Hugh Wang1, Elizabeth Sweeney1, Hang Zhang1,2, Emily B Tanzi1, Jennette Prince2, Victor Antonio Su-Ortiz2, Susan A Gauthier1, and Thanh D Nguyen1
1Radiology, Weill Cornell Medicine, New York, NY, United States, 2Cornell University, Ithaca, NY, United States
Multi-echo T2 relaxometry-based water compartment model can be used to quantify CSF water fraction (CSFF). It turns out that the CSFF increases with age in the cortical gray matter from a cohort of non-active MS subjects. CSFF could be a potential biomarker of perivascular space.
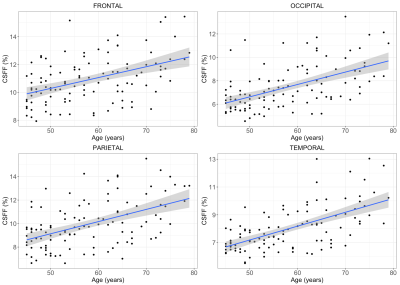
Figure 3 CSFF with age in four cortical lobes. It clearly shows that the CSFF increases with age in all cortical lobes (Frontal: p=0.019, occipital: p<0.01, Parietal: p<0.01, temporal: p<0.01, all p-values are FDR adjusted).
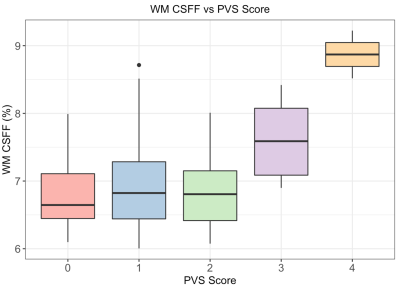
Figure 2. WM CSFF and PVS score relation. PVS score was evaluated on the T2w image at the semiovale slice and the WM CSFF was measured at the WM region of the same slice. It shows CSFF increases as the PVS score increases.
-
Impact of the Acquisition Protocol on the Sensitivity to Demyelination and Axonal Loss of DKI and NODDI: A Simulation Study
Stefania Oliviero1 and Cosimo Del Gratta1
1Department of Neuroscience, Imaging, and Clinical Sciences, University of Chieti Pescara G. D'Annunzio, Chieti, Italy
DKI
metrics significantly changed with axonal loss but not with demyelination,
while NODDI metrics showed sensitivity to both damage processes. In any case, the
sequence strongly affected the sensitivity and, especially for NODDI, the
metric means.
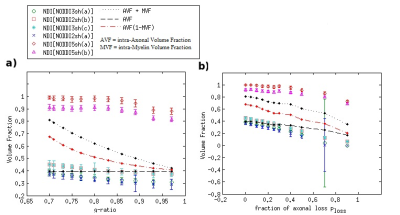
Fig 2 Results for the Neurite Density Index NDI (i.e. the NODDI-derived intra-cellular
fractional volume), using 6 different
acquisition protocols. In a and b, NDI mean values and standard deviations obtained
in different conditions of demyelination and axonal loss, respectively, considering a Rician
noise with SNR=20 affects the synthetic DW signal. For reference, some
specific combinations of the true intra-axonal volume fraction AVF and true
intra-myelin volume fraction MVF are also shown.
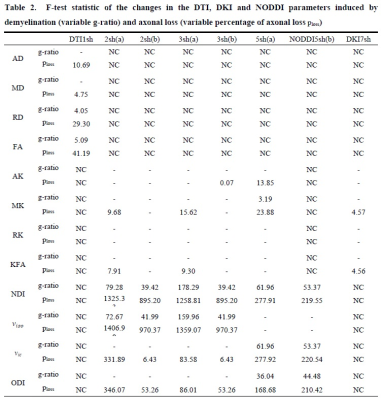
Metrics AD, MD, RD, and FA are DTI-derived; AK,
MK, RK, and KFA are DKI-derived, while neurite density index NDI, fractional
volume
of the isotropic compartment νiso,
fractional volume νic of the intra-cellular
space, and orientation
dispersion index ODI are NODDI-derived. Omitted results (-)
refer to parameters showing non-significant changes between healthy and damaged
condition (one-way ANOVA with p<0.01). NC means Not Calculated, since not all the sequences were used for all
the DWI analyses.
-
Mapping myelin content in ex-vivo MS brain tissue using short-T2 MRI of the lipid-protein bilayer
Emily Louise Baadsvik1, Markus Weiger1, Romain Froidevaux1, Wolfgang Faigle2, Benjamin Victor Ineichen2, and Klaas Paul Pruessmann1
1ETH Zurich and University of Zurich, Zurich, Switzerland, 2University Hospital Zurich and University of Zurich, Zurich, Switzerland
D2O-exchanged
multiple sclerosis brain tissue was imaged with short-T2 MRI
techniques, yielding contrast that is closely correlated with corresponding
myelin-stained cryosections for white matter, grey matter and multiple
sclerosis lesions.
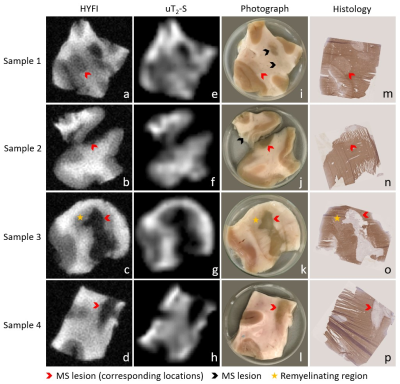
Figure 3: Image series showing
the correlation between MRI results, photographs and histology for all samples.
a-d) High-resolution HYFI images, e-h) amplitude maps of the uT2-S
fit component, i-l) sample photographs, m-p) corresponding 10 µm cryosections with myelin staining using
immunohistochemistry for MOG (brown) and haematoxylin counterstaining (purple).
Note that sample 2 contains two tissue pieces (originally connected), but
histology was only performed on the larger one.
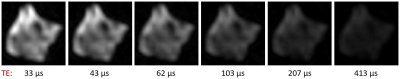
Figure 1: Time evolution of signal
intensity in SPI images of human MS brain tissue after D2O exchange.
Six of the fourteen images acquired for the SPI series of sample 1 are shown.
The signal intensity drops as TE increases, and has all but vanished after 413 μs – a clear indication that the captured signal
stems from tissues with T2 times of around a hundred microseconds at
most.
-
Myelin imaging derived quantitative parameter mapping compared to myelin water fraction
Yuki Kanazawa1, Masafumi Harada1, Yo Taniguchi2, Syun Kitano3, Nagomi Fukuda3, Yuki Matsumoto1, Hiroaki Hayashi4, Kosuke Ito2, Yoshitaka Bito2, and Akihiro Haga1
1Graduate School of Biomedical Sciences, Tokushima University, Tokushima, Japan, 2Healthcare Business Unit, Hitachi, Ltd., Tokyo, Japan, 3Faculty of Medicine, Tokushima University, Tokushima, Japan, 4Faculty of Health Sciences, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University, Kanazawa, Japan
Myelin map derived from QPM can be applied for the quantitative assessment of white matter structure as substitute for MWF.
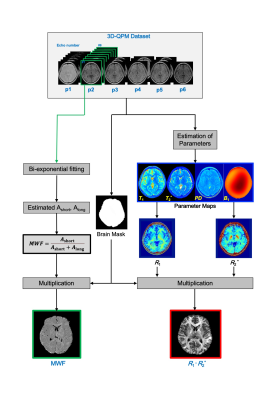
Fig.1 A schematic diagram of the QPM-myelin mapping (R1·R2*) and MWF images procedures.
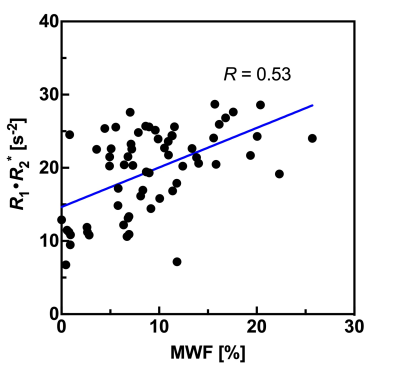
Fig. 5 Plots the relationship between MWF values and R1·R2* product values derived from QPM across all four bilateral structures.
-
Association of estimated time from the onset of multiple sclerosis plaques with myelin and axon-related quantitative MRI measurement
Tomoko Maekawa1,2, Akifumi Hagiwara1,3, Masaaki Hori1,4, Christina Andica1, Shohei Fujita1,5, Toshiaki Akashi1, Koji Kamagata1, Akihiko Wada1, and Shigeki Aoki1
1Department of Radiology, Juntendo University School of Medicine, Tokyo, Japan, 2Division of Regenerative Medicine, The Jikei University School of Medicine, Tokyo, Japan, 3Department of Radiological Science, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, United States, 4Department of Diagnostic Radiology, Toho University Omori Medical Center, Tokyo, Japan, 5Department of Radiology, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan
We investigated the association between estimated time from the onset of multiple sclerosis plaques and myelin- and axon-related quantitative MRI measurements. Multiple sclerosis plaques with longer estimated time from the onset had significantly lower myelin volume fraction.
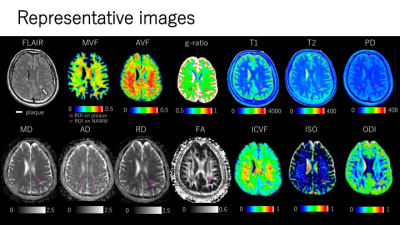
Figure 3. Representative quantitative maps of an MS patient. Plaques were defined as a white matter area of more than 5 mm in diameter, with abnormally high intensity on synthetic FLAIR images. Each plaque was manually segmented. All the ROIs placed on synthetic FLAIR images were copied and pasted onto the quantitative maps.
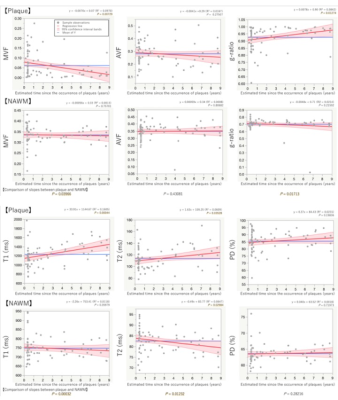
Figure 4. Simple linear regressions to assess the association between estimated time from the onset of plaques and quantitative MRI metrics. Plaque MVF and g-ratio in relation to the estimated time from the onset were significantly fitted to linear lines with negative and positive slopes, respectively. Plaque T1 and T2 in relation to the estimated time from the onset were significantly fitted to linear lines with positive slopes. NAWM T2 in relation to the estimated time from the onset was significantly fitted to a linear lineswith a negative slope.