-
Determining Spinal Cord pH using Chemical Exchange Saturation Transfer (CEST) MRI
Alicia Cronin1,2, Patrick Liebig3, Sarah Detombe4, Neil Duggal4, and Robert Bartha1,2
1Medical Biophysics, University of Western Ontario, London, ON, Canada, 2Centre for Functional and Metabolic Mapping, Robarts Research Institute, London, ON, Canada, 3Siemens Healthineers, Erlangen, Germany, 4Clinical Neurological Sciences, University Hospital, London Health Sciences Centre, London, ON, Canada
A prototype 2D gradient echo CEST
sequence utilizing a gradient-echo readout with centric reordering on a
Siemens scanner was utilized in conjunction with a respiratory correction
method to improve amide proton CEST contrast at 3.0 T in the spinal cord.
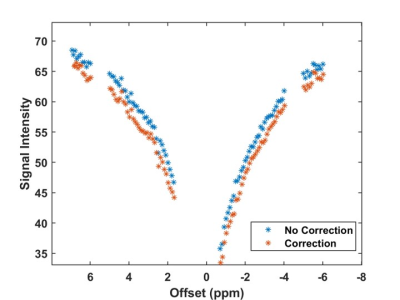
Figure 2:
Demonstration of
the respiratory correction in a full CEST spectrum collected in the spinal cord
with no correction (blue) and implemented correction (orange) demonstrated.
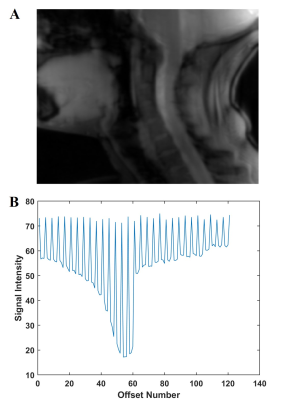
Figure
1:
A: Raw sagittal spinal cord CEST image.
B: CEST spectrum of spinal cord with the interleaved non-saturated scans
demonstrating the global respiratory effect.
-
The Added Value of Inflow-Based Vascular-Space-Occupancy and Diffusion-Weighted Imaging in Preoperative Grading of Gliomas
Haimei Cao1, Xiang Xiao1, Jun Hua2,3, Guanglong Huang4, Xiaodan Li1, Wenle He1, Jie Qin1, and Yuankui Wu1
1Department of Medical Imaging, Nanfang Hospital, Southern Medical University, Guangzhou, China, 2Neurosection, Division of MRI Research, Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4Department of Neurosurgery, Nanfang Hospital, Southern Medical University, Guangzhou, China
Both iVASO-rCBVa
and DWI-mADC can predict gliomas grades and combining these two parameters can
further improve diagnostic performance. Also, VASO and DWI have the added value
to structual MRI in preoperative prediction of tumor grading of gliomas.
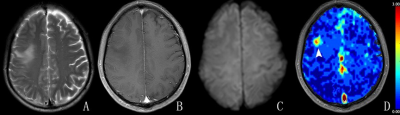
Figure
2. Right frontal lobe anaplastic oligodendroglioma in a 51-year-old male (Grade
III). The lesion in the right frontal lobe was iso-intense with perilesional
edema on T2WI (A) and showed no enhancement on contrast-enhanced T1WI (B). The
lesion showed an iso-intensity on diffusion-weighted image (C) and the focus of
elevated arteriolar perfusion (arrowhead) on CBVa map (D). On review 1, the
lesion was diagnosed as a low-grade glioma. On review 2, the lesion was
diagnosed as a high-grade glioma.
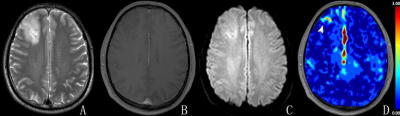
Figure 1. Right frontal lobe anaplastic astrocytoma
(Grade III) in a 39-year-old male. The lesion in the right frontal lobe was
hyper-intense on T2WI (A) and showed no enhancement on contrast-enhanced T1WI
(B). The lesion showed a slight hyper-intensity on diffusion-weighted image (C)
and the focus of elevated arteriolar perfusion (arrowhead) on CBVa map (D). On
review 1, the lesion was diagnosed as a low-grade glioma. On review 2, the
lesion was diagnosed as a high-grade glioma.
-
Alterations in brain connectivity as duration of disease increases in Parkinson's disease.
Priyanka Bhat1, S Senthil Kumaran2, Achal K Srivastava1, and Vinay Goyal3
1Neurology, AIIMS, Delhi, India, 2Nuclear Magnetic Resonance, AIIMS, Delhi, India, 3Neurology, Medanta, The Medicity, Gurgaon, India
A functional connectivity study revealing altered cortico-subcortical connectivity with progressively declining motor function in Parkinson's disease as the duration of the disease increases.
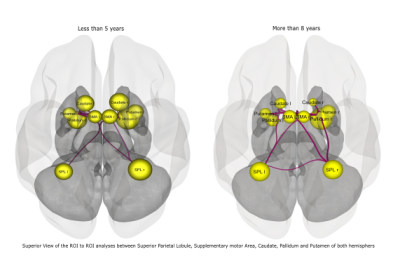
Figure 2: ROI to ROI connectivity between the 3 groups : A- Less than 5 years B- 5 to 8 years C- More than 8 years.
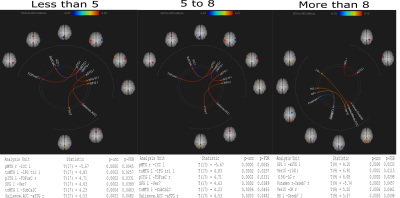
Figure 1: Seed to seed connectivity between the 3 groups :
A-
Less than 5
years
B-
5 to 8 years
C-
More than 8
years.
-
DTI and NODDI assessment of posterior optic pathway function in sellar and parasellar tumor patients
Eun-Jung Choi1, Koung Mi Kang2, Woojin Jung1, Jongho Lee1, Seung Hong Choi2, and Yong Hwy Kim3
1Department of Electrical and Computer Engineering, Seoul National University, Seoul, Korea, Republic of, 2Department of Radiology, Seoul National University Hospital, Seoul, Korea, Republic of, 3Department of Neurosurgery, Seoul National University Hospital, Seoul, Korea, Republic of
DTI and NODDI parameters
in the optic radiations were significantly correlated with a preoperative
visual field impairment score and associated with improvement of postoperative
visual field impairment in patients with the compression of optic chiasm.
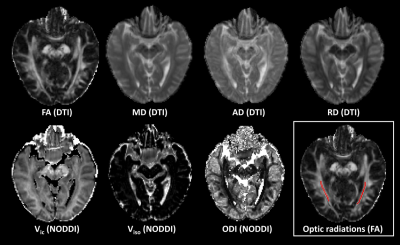
Figure 1. Representative DTI and NODDI parameter maps. The each side optic radiation
was drawn on the FA map.
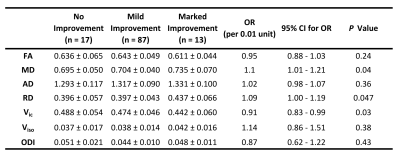
Figure 4. DTI
and NODDI Parameters and Postoperative Visual Field Improvement. Ordinal
logistic regression was performed using 3 ordinal outcomes (no improvement or
worse after surgery, Δ VFIS = 0 ~ 4; mild improvement after surgery, Δ VFIS =
-4 ~ -1; and marked improvement after surgery, Δ VFIS = -8 ~ -5).
-
White Matter in Metachromatic Leukodystrophy as Assessed by Myelin Water Fraction and Diffusion Tensor Imaging
Laleh Eskandarian1,2, Safak Parlak3, Onur Afacan4,5, Ceren Günbey6, Nesibe Gevher Ertuğrul6, Banu Anlar6, and Kader Karli Oguz2,3
1Neuroscience Department, Bilkent University, Ankara, Turkey, 2National Magnetic Resonance Research Center (UMRAM), Bilkent University, Ankara, Turkey, 3Faculty of Medicine, Department of Radiology, Hacettepe University, Ankara, Turkey, 4Department of Radiology, Boston Children’s Hospital, Boston, MA, United States, 5Department of Radiology, Harvard Medical School, Boston, MA, United States, 6Department of Pediatrics, Hacettepe University, Ankara, Turkey
MLD is a dysmyelinating autosomal recessive lysosomal storage disease. T2WI may not show the disease involvement accurately, especially in early phases where a bone marrow transplant can be a treatment option. We used Myelin Water Fraction and DTI to investigate WM in patients with MLD.
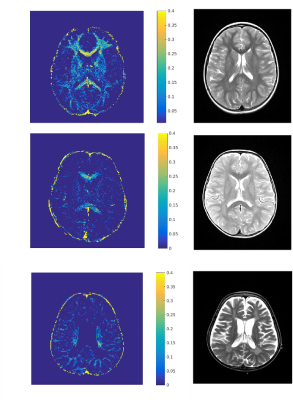
Figure 1. Axial T2WI and
T2 MWF maps
of a
7-year old female HC (A)
and
two
patients with MLD, 4 and 9-years old at
(B, C respectively)
with
varying
severity
of leukodystrophy.
Both
patients
have
reduced
MWF compared
with
HC in A. Although
slight
hyperintensity
is noticed
on T2WI; the
patients
have
markedly
low
myelin
on MWF.
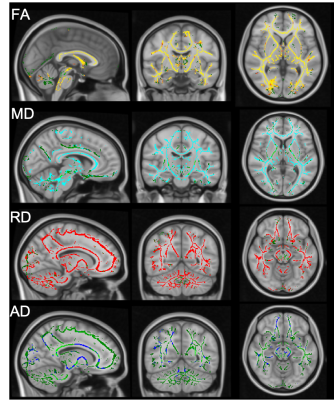
Figure 2. Shows TBSS maps
derived
from
DTI studies
from
the
patients
and
HCs.
Compared
with
HCs,
FA map
shows
widespread
significant
reduction
(A), MD and
RD maps
show
increase
(B and
C ) and
AD map
shows
a limited
reduction
in WM of the
patients.
-
Exploring edematous nerve fibers by using Neurite Orientation Dispersion and Density Imaging
Shin Tai Chong1, Xinrui Liu2, Hung-Wen Kao3, Chien-Yuan Eddy Lin4, Sanford PC Hsu5, and Ching-Po Lin1
1Institute of Neuroscience, National Yang-Ming University, Taipei, Taiwan, 2Department of Neurosurgery, First Hospital of Jilin University, Jilin, China, 3Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan, 4GE Healthcare, Taipei, Taiwan, 5Department of Neurosurgery, Neurological Institute, Taipei Veterans General Hospital, Taipei, Taiwan
Neurite Orientation Dispersion and Density Imaging (NODDI)-based tractography could improve the reconstruction of the fiber tracts that by tracking through regions of peritumoral edema.
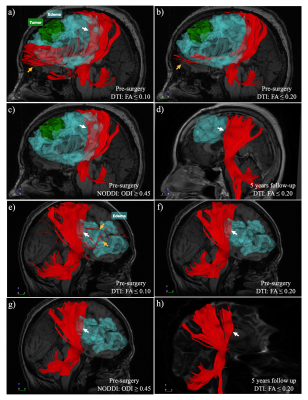
Two examples (a-d, meningioma, WHO grade II; e-h, malignant B-cell lymphoma) of visual comparison between DTI and NODDI-based CST reconstruction. NODDI-based tractography with ODI has better performance in the region of peritumoral edema and fewer false-positive tracts (c & g, white arrow). After the vasogenic edema subsided, the DTI-based tractography with FA ≤ 0.2 shows CST similar to the pre-surgery NODDI-based CST (d & h).
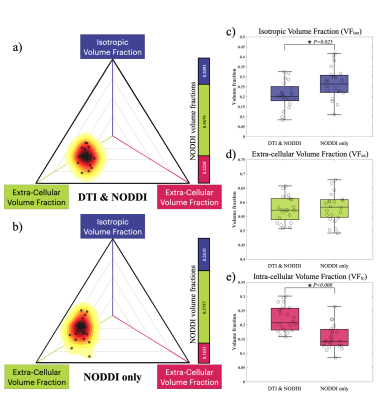
NODDI's volume fraction distributions in the DN group and N group are plotted with a 2D barycentric coordinate system. As compared with the DN group (a), the N group (b) distribution shows a left upper shift in the coordinate system. There is no significant difference in VFec (P = 0.733, d), but significant differences in VFic (P < 0.001, e) and VFiso (P = 0.025, c).
-
Assessment of IDH1 Mutation Status and MGMT Promoter Methylation Status of Gliomas Using DWI, IVIM and DKI
Yan Xie1, Shihui Li1, Nanxi Shen1, Weiyin Vivian Liu2, and Wenzhen Zhu1
1Department of Radiology, Tongji Hospital, Tongji Medical College, HUST, Wuhan, China, 2MR Research, GE Healthcare, Beijing, China
Diffusion MRI parameters were able to significantly distinguish the
mutation state of IDH1 in low-grade
gliomas, as well as the methylation state of the MGMT promoter, but not
in glioblastomas.
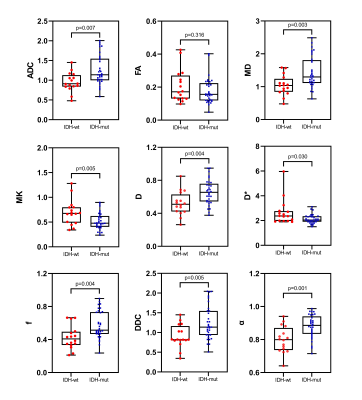
Figure 3 Box and whisker plots
of DWI, IVIM and DKI metrics in lower-grade
gliomas stratified according to IDH1 genotype (IDH1 mutant and IDH1 wild type).
Boxes represent the median ± quartiles, with whiskers extending to the maximum
and minimum values.
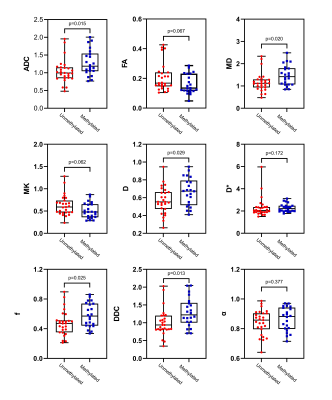
Figure 4 Box and whisker plots
of DWI, IVIM and DKI metrics in lower-grade
gliomas stratified according to MGMT promoter status (methylated and unmethylated).
Boxes represent the median ± quartiles, with whiskers extending to the maximum
and minimum values.
-
NODDI in detecting cognitive decline in patients with radiation-induced brain injury: comparison with DTI
Weike Zeng1 and Mengzhu Wang2
1Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China, 2MR Scientific Marketing, Siemens Healthcare, Guangzhou, China
Decreased ICVF value in brain edema lesion indicated the reduction of neurite density, and was associated with cognitive decline in RI. NODDI as a new MRI diffusion technique may contribute to better understanding of pathophysiology of cognitive decline in RI than DTI.
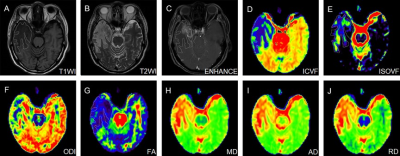
Figure 1. NODDI maps of a patient with RI, together with DTI maps, T1WI, T2WI and enhanced images. RI, radiation-induced brain injury; T1WI, T1-weighted imaging; T2WI, T2-weighted imaging; FA, fractional anisotropy; MD, mean diffusivity; AD, axial diffusivity; RD, radial diffusivity; ICVF, intra-neurite volume fraction; ODI, orientation dispersion index; ISOVF, volume fraction of the isotropic compartment.
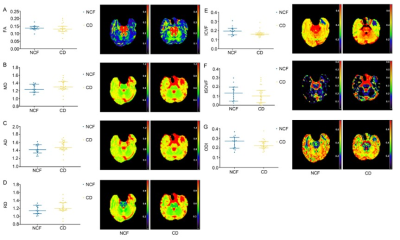
Figure 2. Differences of DTI parameters including FA (A), MD (B), RD (C) and MD (D) as well as NODDI parameters including ICVF (E), ISOVF (F) and ODI (G) of edema lesions between patients with or without cognitive decline in RI. RI, radiation- induced brain injury; NCF, normal cognitive function; CD, cognitive decline; FA, fractional anisotropy; MD, mean diffusivity. AD, axial diffusivity; RD, radial diffusivity; ICVF, intra-neurite volume fraction; ODI, orientation dispersion index; ISOVF, volume fraction of the isotropic compartment.
-
Post-surgery network reorganization in glioma patients: a longitudinal study of functional segregation and centrality
Beatrice Federica Luciani1, Francesca Saviola1, Luca Zigiotto2,3, Stefano Tambalo1, Domenico Zacà1, Lisa Novello1, Silvio Sarubbo2,3, and Jorge Jovicich1
1CIMeC Center for Mind/Brain Sciences, University of Trento, Rovereto (Trento), Italy, 2Department of Neuroscience, Division of Neurosurgery, S.Chiara Hospital, APSS, Trento, Italy, 3Structural and Functional Connectivity Lab, S.Chiara Hospital, APSS, Trento, Italy
Left-lateralized gliomas and high-grade gliomas reduce longitudinal functional segregation and centrality after surgical resection. The Default Mode Network supports post-surgical plasticity, highlighting its relevance for pre-surgical planning.
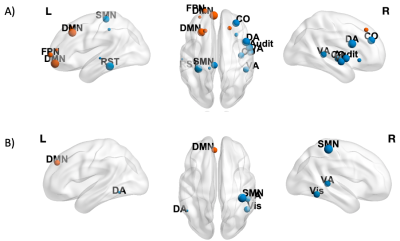
Nodes showing a significant negative effect (β<0, p<0.05 FDR-corrected) of Time on CI (A) and BC (B) are shown in blue (non-hubs) and orange (hubs). Nodes are weighted for the statistical significance (p-value). For image display purposes, concerning CI, labels are plotted for most significant nodes (threshold 0.03).
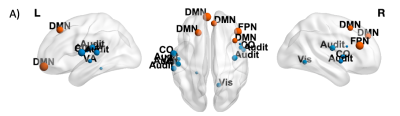
Nodes showing a significant negative effect (β<0, p<0.05 FDR-corrected) of Time × Tumor grade on Cl in LGG patients are represented in blue (non-hubs) and orange (hubs). Nodes are weighted for the statistical significance (p-value). For image display purposes, concerning Cl, labels are plotted for most significant nodes (threshold 0.03).
-
Fast 3D Wave-CAIPI Susceptibility Weighted Imaging and SPACE FLAIR for Comprehensive Evaluation of Demyelinating Lesions in Multiple Sclerosis
Augusto Lio M. Goncalves Filho1,2, Azadeh Tabari1,2, Chanon Ngamsombat3, Ilena George4, Stephen F. Cauley2, Wei Liu5, Daniel N. Splitthoff6, Wei-Ching Lo7, Pamela W. Schaefer1, Otto Rapalino1, Eric C. Klawiter4, John Conklin1,2, and Susie Y. Huang1,2
1Department of Radiology, Massachusetts General Hospital, Boston, MA, United States, 2Department of Radiology, Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 3Department of Radiology, Siriraj Hospital, Bangkok, Thailand, 4Department of Neurology, Massachusetts General Hospital, Boston, MA, United States, 5Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China, 6Siemens Healthcare GmbH, Erlangen, Germany, 7Siemens Medical Solutions Inc., Boston, MA, United States
Accelerated Wave-CAIPI susceptibility weighted imaging (SWI) and
FLAIR may improve the characterization of demyelinating lesions within
reasonable acquisition times and provide a more confident diagnosis of multiple
sclerosis in brain MRI at 3T.
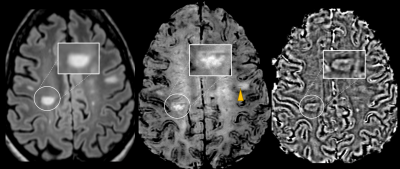
Figure 1. Axial Wave-SPACE-FLAIR
(left), Wave-SWI (middle), and phase images (right) in a 28-year-old
patient with confirmed MS demonstrating the presence of paramagnetic rims
corresponding to lesions visible on FLAIR (highlighted). A lesion with a central
vein sign can be seen in the same Wave-SWI sequence (arrowhead).
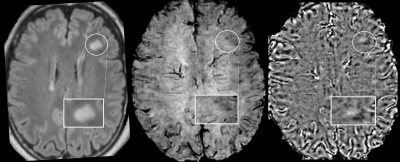
Figure 3. Axial Wave-SPACE-FLAIR
(left), Wave-SWI (middle), and phase images (right) in a 20-year-old
patient with confirmed anti-MOG disease with no visible paramagnetic rim around
the lesion visible on FLAIR (highlighted). No lesions with a central vein sign were
identified on the Wave-FLAIR and Wave-SWI sequences.
-
Glioblastoma Recurrence vs. Radiotherapy Injury: Combined Model of DKI and 11C-MET Using PET/MR May Increase Accuracy of Differentiation
Haodan Dang1, Ruimin Wang1, Jiajin Liu1, Huaping Fu1, Mu Lin2, Jiahe Tian1, Jinming Zhang1, and Baixuan Xu1
1Department of nuclear medicine, Chinese PLA General Hospital, Beijing, China, 22. MR Collaboration, Diagnostic Imaging, Siemens Healthcare, Shanghai, China
DKI, 11C-MET PET and
histogram parameters provide complementary information about tissue. The
decision-tree model combined of theses parameters has the potential to further
increase diagnostic accuracy. 11C-MET PET/MR
may thus contribute to the management of glioblastoma patients.
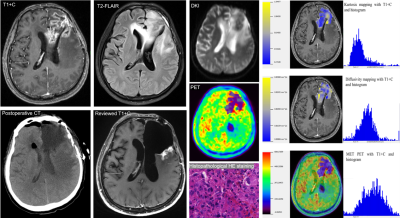
Figure 1. The PET/MR
images of glioblastoma recurrence.
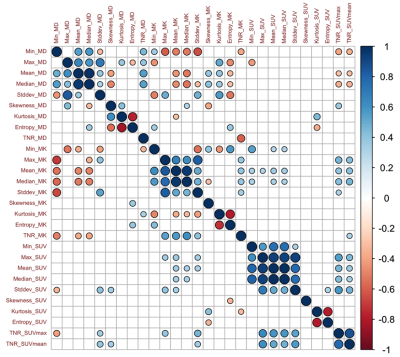
Figure 2. Correlation matrix of each parameter pair with significance
levels for glioblastoma recurrence (right upper part) and radiotherapy injury
(left lower part) groups.
-
Usefulness of Quantitative Susceptibility MRI for the Detection of Iron in the Motor Cortex in Amyotrophic Lateral Sclerosis
qianwen li1, juan Wei2, and jie Lu1
1Xuanwu Hospital,Capital Medical University, Beijing, China, 2GE Healthcare, Beijing, China
Quantitative Susceptibility Mapping (QSM), a newly developed quantitative and accurate measurement method that can be used to detect iron-related motor cortex alterations in patients of Amyotrophic lateral sclerosis (ALS) that may be relevant to pathologic changes.
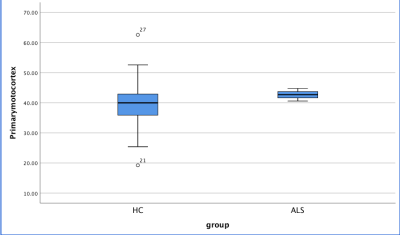
Figure1.The susceptibility of primary motor cortex of ALS patients were significantly higher than those of controls
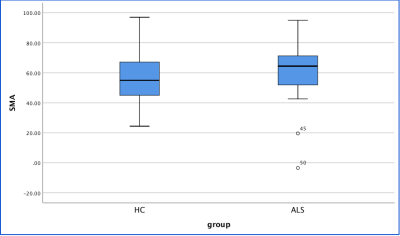
Figure 2.The susceptibility of SMA of ALS patients were significantly higher than those of controls
-
Disturbed interhemispheric functional and structural connectivity in Type 2 diabetes
Ying Cui1, Tian-yu Tang2, and Shenghong Ju1
1Department of Radiology, Zhongda hospital, Southeast University, Nanjing, China, 2Southeast University, Nanjing, China
T2DM patients showed disrupted interhemispheric
coordination, especially in the occipital lobe. These disruptions are strongly
correlated with insulin resistance, which might be an important treatment
target to abate the cognitive decline in diabetic subjects.
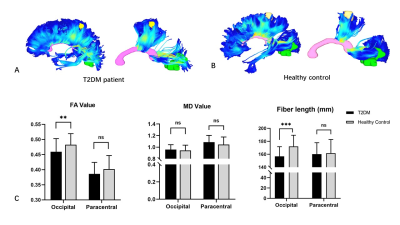
Figure 2. Tractography
results in representative subjects and group differences. The fibers connecting bilateral occipital lobes
in T2DM representative (A) were slender and more diffusely orientated compared
with the healthy controls (B). ** indicates P <0.01, *** indicates P
<0.001.
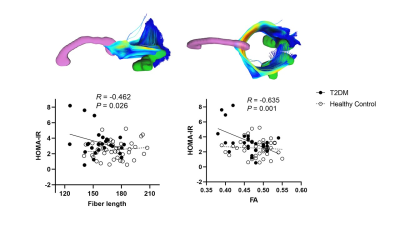
Figure 3. Correlations between insulin
resistance and the diffusion measures of the occipital fibers. Note
that no such correlations were observed in the
control group. The first row displays the occipital fibers derived from the HCP1021-1mm
template (https://pitt.app.box.com/v/HCP1021-1mm).
-
Noninvasive assessment of MGMT promoter methylation status in grade II-IV gliomas using inflow-based vascular-space-occupancy
Yuankui Wu1, Wenle He1,2, Wensheng Wang2, Jun Hua3,4, Xiang Xiao1, Xiaomin Liu1, Yikai Xu1, and Yingjie Mei5
1Department of Medical Imaging, Nanfang Hospital, Southern Medical University, Guangzhou, China, 2Department of Radiology, Guangdong 999 Brain Hospital, Guangzhou, China, 3Neurosection, Division of MRI Research, Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 5Philips healthcare, Guangzhou, China
The top two CBVa histogram features in predicting MGMT promoter methylation
in grade II – IV gliomas were RMS & Varianc. Combing CBVa histogram
features and structural MRI features improved diagnostic performance.
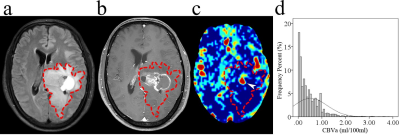
Figure
1. Glioblastoma with MGMT promoter methylation in a 39-year-old male. (a) Axial
T2-FLAIR image. (b) Axial postcontrast T1-weighted image. (c) Axial CBVa map
(ml/100 ml). (d) Histogram of CBVa from whole tumor ROI (dashed line). Images
show a left thalamic mass with cystic change, which involved the lateral
ventricular walls but spared the cortices. The CBVa map shows focal
hyperperfusion (arrowhead) in the mass. The corresponding histogram shows a narrow
distribution of perfusion in the whole tumor which is mainly concentrated in hypoperfused
areas.
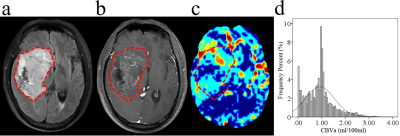
Figure 2. Glioblastoma
without MGMT promoter methylation in a 44-year-old male. (a) Axial T2-FLAIR
image. (b) Axial postcontrast T1-weighted image. (c) Axial CBVa map (ml/100
ml). (d) Histogram of CBVa from whole tumor ROI (dashed line). The images
show an inhomogeneous mass in the right temporal lobe, which invaded the right
basal ganglia, the cortices, and the subventricular zone. The corresponding
histogram shows a wide distribution of perfusion in the whole tumor, with a
peak located at around 1.0 ml/100 ml.
-
Susceptibility-Based Characterization of Venous Distribution and Oxygen Extraction Fraction in Brains with Glioma at 7T
Shihui Zhou1,2, Huilou Liang2,3, Yuchao Liang4, Siqi Cai1,2, Chunxiang Jiang1,2, Rong Xue2,3, Lei Wang4, and Lijuan Zhang*1
1Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2University of Chinese Academy of Sciences, Beijing, China, 3State Key Laboratory of Brain and Cognitive Science, Beijing MRI Center for Brain Research, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China, 4Neurosurgery, Beijing Tiantan Hospital of Capital Medical University, Beijing, China
Venous and OEF distribution of the brain varies with the malignant grade of glioma. Low grade gliomas induce more extensive interhemispheric difference in venous and OEF distribution. Oxygenation changes were found coupled with altered venous distribution in brains with high grade glioma.
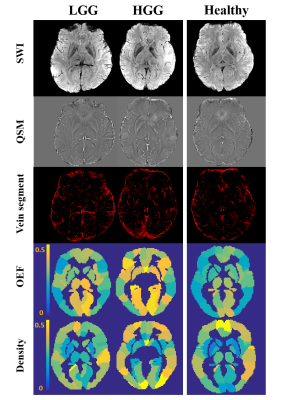
Figure
1. Maps of
vein density and OEF for representative low grade (LGG), high grade glioma (HGG) cases and control subject.
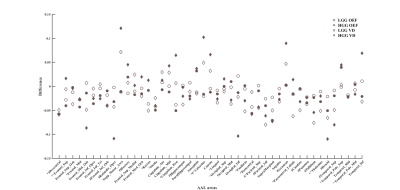
Figure
2. Differential venous and OEF distribution
based on AAL atlas. # *indicate significant interhemispheric
difference of vein density(VD) and OEF in LGGs; ^ + indicate significant interhemispheric difference of VD and OEF in HGGs, resepectively (paired t
test, p<0.05, FDR corrected).
-
Tau-mediated microstructural changes in the central tegmental tract in APOE-ε4 positive mild cognitive impairment
Jason Langley1, Sana Hussain2, Daniel E Huddleston3, Ilana Bennett4, and Xiaoping P Hu1,2
1Center for Advanced Neuroimaging, University of California Riverside, Riverside, CA, United States, 2Department of Bioengineering, University of California Riverside, Riverside, CA, United States, 3Department of Neurology, Emory University, Atlanta, GA, United States, 4Department of Psychology, University of California Riverside, Riverside, CA, United States
Locus coeruleus axons
project to the thalamus via the central tegmental tract (CTT). In the APOE-e4 positive group, CTT
microstructural measures were positively correlated with thalamus
tau-PET SUVR but no correlations were observed in the APOE-e4 negative group.
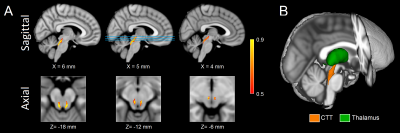
Figure 1. Sagittal (A; top row)
and axial (A; bottom row) of population maps showing the CTT ROI used in this
analysis. The lines in sagittal view at X=5 mm identify the location of the axial
slices shown in the bottom row in MNI space. A three dimensional rendering of the CTT is
shown in B.
CTT - central tegmental tract; ROI - region of interest; MNI - Montreal Neurological Institute
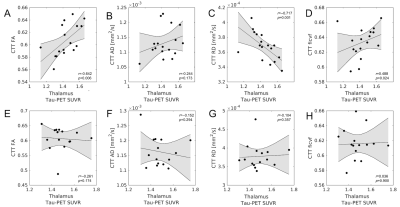
Figure 3. Correlations between CTT microstructure metrics and thalamus
tau-PET SUVR in APOE-ε4 positive subjects (top row; A-D) and APOE-ε4 negative subjects (bottom row; E-H). Significant
correlations between CTT microstructure and thalamus tau-PET SUVR are seen in
the APOE-ε4 positive group but not in the APOE-ε4 negative group.
-
Joint and individual statistical analysis of brain MRI and cognition measures in Alzheimer's Disease
Raphiel Jamale Murden1, Deqiang Qiu2, and Benjamin B Risk2
1Biostatistics and Bioinformatics, Emory University, Atlanta, GA, United States, 2Emory University, Atlanta, GA, United States
Canonical Joint and Individual Variation Explained incoroporates a step to impute missing data and uses a computationally efficient permutation test to estimate the joint rank. Patterns of variation shared between measures of brain atrophy and cognition areassociated with diagnoses.
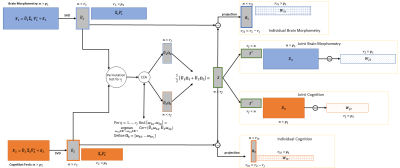
Schematic of the CJIVE process. Singular value decomposition (SVD) is applied to the input data matrices (far left) to obtain dimension-reduced PC scores. Next, a permutation test is used to determine the number of components that will lie in the joint subspace. CCA is applied to the PC scores and a weighted average of canonical variables determines the joint subspace. Finally, individual subspaces can be determined by the orthogonal complement of the joint subspace within the respective PC subspaces.
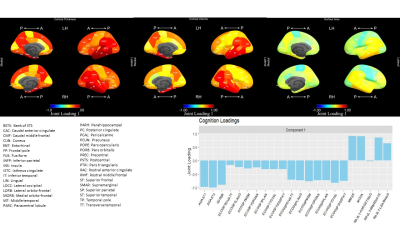
Joint Loadings for component 1. Temporal lobe ROI measures were most prominent among morphometry loadings, including both thickness and volume. ADAS and MMSE dominated cognition loadings (bottom) and both have been shown as excellent diagnostic tools. The proportions of total variation explained by the first joint component were 0.147 for morphometry and 0.467 for cognition. Proportions explained by the second component (not shown) were 0.025 and 0.044 for morphometry and cognition, respectively.
-
Functional connectivity-based prediction of Autism on site harmonized ABIDE dataset
Madhura Ingalhalikar1, Sumeet Shinde1, Arnav Karmarkar1, Archith Rajan1, Rangaprakash D2, and Gopikrishna Deshpande3
1Symbiosis Centre for medical image analysis, Symbiosis international university, Pune, India, 2Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, MA, United States, 3Department of Electrical and Computer Engineering, Auburn University, Auburn, AL, United States
Superior
prediction of Autism on ABIDE dataset is demonstrated as we include
site-harmonization before applying machine learning algorithms. The ablation
analysis provides sub-network based interpretability.
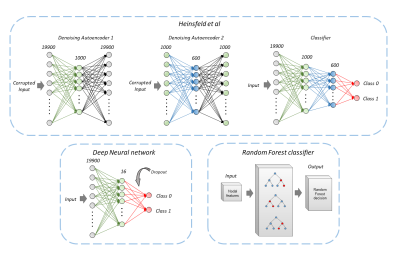
Figure 1: Schematic diagram of all the classification
methods used. An artificial neural network (ANN) based classifier was
implemented along with a Random forest (RF) of classification trees.
Architecture for classification involving denoising autoencoders based on
Heinsfeld et al has been shown.
Figure 2: Brain
maps showing ROIs associated with each of the 12 networks used in ablation
analysis
-
Ultra-High Field Sodium MRI in Alzheimer’s Disease Reveals Stage-dependent Metabolic Alterations Associated with Tau-pathology
Alexa Haeger1,2,3, Michel Bottlaender1,4, Julien Lagarde4,5,6, Renata Porciuncula Baptista1, Cécile Rabrait-Lerman1, Volker Luecken2,3, Jörg Bernhard Schulz2,3, Alexandre Vignaud1, Marie Sarazin4,5,6, Kathrin Reetz2,3, Sandro Romanzetti2,3, and Fawzi Boumezbeur1
1BAOBAB, CNRS, Paris-Saclay University, CEA-NeuroSpin, Gif-sur-Yvette, France, 2Department of Neurology, RWTH Aachen University, Aachen, Germany, 3JARA-BRAIN Institute of Molecular Neuroscience and Neuroimaging, Forschungszentrum Jülich GmbH, Julich, Germany, 4BioMaps, CNRS, Inserm, Paris-Saclay University, CEA-SHFJ, Orsay, France, 5Neurology of Memory and Language, GHU Paris Psychiatrie & Neurosciences, Sainte-Anne Hospital, Paris, France, 6Université de Paris, Paris, France
By combining quantitative 23Na MRI at 7
Tesla with Tau/Amyloid-PET, we show that total sodium concentration is
increased in AD patients compared to controls and that these changes are more strongly
correlated with local Tau- than Amyloid-loads.
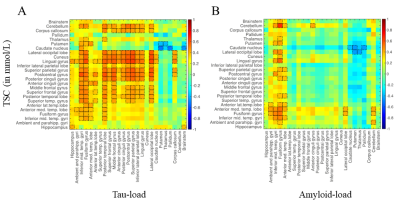
Figure 4. Correlation matrices between TSC versus local Tau
load (A) and versus local Amyloid load (B). Notable correlations
are reported at an uncorrected p < 0.05 level of significance and are framed
in black.
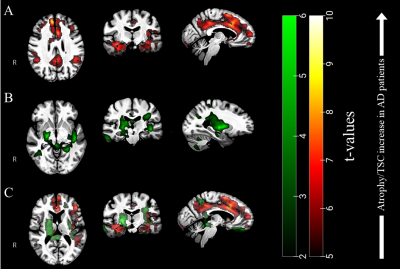
Figure 3. (A) T-values resulting
from voxel-based permutation analyses of our TSC maps (AD > Controls) and (B) atrophy clusters in green (Controls
> AD), overlaid to our group template. (C) From the overlay of both TSC and
atrophy clusters, one can appreciate the differences between both pathological patterns.
-
Feasibility of Arterial Spin Labeling for Detection of Longitudinal Changes in Perfusion in Elderly and Frontotemporal Dementia Patients
Tracy Ssali1,2, Lucas Narciso1,2, Matthais Günther3, Frank Prato1,2, Udunna Anazodo1,2, Elizabeth Finger4, and Keith St Lawrence1,2
1Lawson Health Research Institute, London, ON, Canada, 2Department of Medical Biophysics, Western University, London, ON, Canada, 3Fraunhofer Institute for Medical Image Computing MEVIS, Bremen, Germany, 4Department of Clinical Neurological Sciences, Western University, London, ON, Canada
Longitudinal variability in perfusion among frontotemporal dementia patients and healthy controls was assessed on a voxel-by-voxel basis over a month. Power analysis revealed ASL has the sensitivity to longitudinal changes as low as 7-8%.
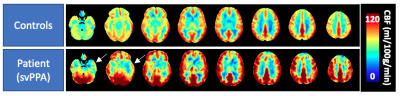
Figure 1: Perfusion averaged over all healthy controls and an exemplary dementia patient participant with semantic variant primary progressive aphasia (svPPA). White arrows indicate regional perfusion deficits.
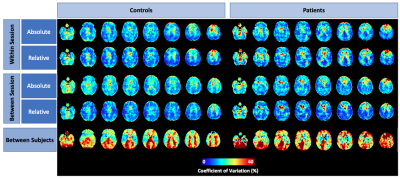
Figure 3: Within-session and between-session coefficient of variation maps in healthy controls and FTD patients using absolute and relative perfusion.
